��Ŀ����
����Ŀ��ij���������ú�ˮ��Դ���������������Ʊ����������ͼ״����������仯���������£�
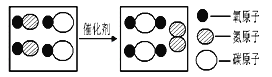
��֪��2H2��CO![]() CH3OH
CH3OH
�ش��������⣺
��1�����ʳ��ˮ�Ļ�ѧ����ʽΪ�� ����ʵ���ҽ��е��ˮ��ʵ���У�Ϊ����ǿ�����ԣ�������ˮ�м�������ϡ���ᣬ�����ܼ����Ȼ����������� ��
��2�����ù���ÿ�������80���״�(CH3OH)����Ҫ�ṩ ��������
��3���ù���ÿ����80���״���Ҫ���Ķ��ٶ�26%���Ȼ�����Һ��(��3��д���������)
���𰸡���1��2NaCl +2H2ͨ��2NaOH + H2��+ Cl2��
���ʳ��ˮ�ò���������������������
��2��10 ��3��2250 ��
��������
�����������1��������������ͼ��֪�����ʳ��ˮ�õ�NaOH ��H2��Cl2�������ʳ��ˮ�Ļ�ѧ����ʽΪ��2NaCl +2H2O![]() 2NaOH + H2��+ Cl2������ʵ���ҽ��е��ˮ��ʵ���У�Ϊ����ǿ�����ԣ�������ˮ�м�������ϡ���ᣬ�����ܼ����Ȼ����������������ʳ��ˮ�ò���������������������
2NaOH + H2��+ Cl2������ʵ���ҽ��е��ˮ��ʵ���У�Ϊ����ǿ�����ԣ�������ˮ�м�������ϡ���ᣬ�����ܼ����Ȼ����������������ʳ��ˮ�ò���������������������
��2�����ݻ�ѧ��Ӧǰ��Ԫ�ص��������䣬�ʿ����ñ���ʽ��Ԫ������=������������Ԫ�ص���������������������=80����4/32��100%=10��
��3�����ݻ�ѧ����ʽ��NaCl + 2H2Oͨ��2NaOH + Cl2��+ H2����H2��NaCl��������ϵ�������NaCl����������һ���ɼ����Ȼ�����Һ������
����������10��������Ҫ�Ȼ��Ƶ�����Ϊx
2NaCl + 2H2O![]() 2NaOH + Cl2�� + H2��
2NaOH + Cl2�� + H2��
117 2
x 10t
117/ x = 2/ 10 t
x = 585 t
��Ҫ26%��ʳ��ˮ������Ϊ585 t��26%��2250 t
���ù���ÿ����80���״���Ҫ����2250 ��26%���Ȼ�����Һ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�