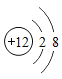��Ŀ����
����Ŀ�����ڣ�С����ĸ�����������ζ����̫����ܰ�����⣬���ܴ���Ȼ������Ϊ�����֡���̼���С�������ڽӽ�Ŀ�ĵ�ʱ�����Ǹ��ﹲ������������ѧ���Ļ�ѧ֪ʶ������������������������⡣

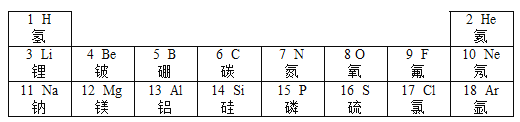
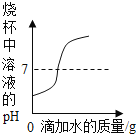
��1����ͼ��ʾ���г��еIJ������ڽ������ϵ���_______������ţ���ͬ���������л��ϳɲ��ϵ���_____________��
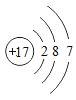
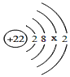
��2�������ѺϽܵġ�����������������㣬����ǿ�ȸߡ���ԭ�ӵĽṹʾ��ͼ������x=___________��
��3������ʵ����̥�ġ���������������Ч��ֹ��̥������ͨ���г������ױ�̥�����÷��ӵĹ۵���͡������ױ�̥����ԭ��________________��
��4��ɨ���ά��������á���������������ά�����������һ������PVC���ɽ���ֽ��PVC[��ѧʽ(C2H3Cl)n]��̼Ԫ�غ���Ԫ�ص�������Ϊ_______________��
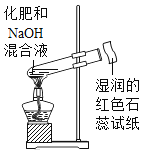
��5�����ǽ��뾰������ɽ���Ͽ���һ���������͵IJ�Ҷ�����·�������IJ�Ҷ���˱Ƕ�����Ա������С��Ϊʹ��Ҷ���ܸ��õĿ����溦�����Ӳ�Ҷ���ճɣ�����ʩ����һ�ָ��Ϸ���________________��(����ţ���ͬ)
ANH4HCO3 BKCl CNH4H2PO4 DKNO3
��6��������ɽ�������˼���ͣ�ʳƷ��������㳦��ƻ�����߸�ţ�̣�����ƻ����Ҫ�ܲ����Ӫ������____________��
��7��������ɽ;�У�С��ʰȡ��һЩ�������ο����ӵĿ�Ȫˮƿ�������ޡ����ϴ�����Ʊ������ͼ����Ʒ�������ǷŴ�λ�õ���Դ�����б�ʾ��ѭ�����õ�ͼ����___________��

���𰸡��ڢ� �٢� 10 �¶����߷��Ӽ������ 8:1 D ά���� B
��������
��1���������ϰ��������ͺϽ�����ͭ˿���ѺϽ����ڽ������ϣ������л��ϳɲ��ϰ��������ϡ��ϳ���ά���ϳ�����������̥���ֱ��������л��ϳɲ��ϣ�����ڢۣ��٢ܣ�
��2��ԭ���У�������=���������������22=2+8+x+2��x=10�����10��
��3�����죬�¶����ߣ����ӵ����������˶����ʼӿ죬�����������ױ�̥������¶����߷��Ӽ������
��4�����ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ���PVC[��ѧʽ��C2H3Cl��n]��̼����Ԫ�ص�������=[n��12��2��]��[n��1��3��]=8��1�����8��1;
��5�������溦�����Ӳ�Ҷ���ճɵĸ��Ϸ���Ӧ���е�Ԫ�غͼ�Ԫ�ء�
A��NH4HCO3�к��е�Ԫ�أ����ڵ��ʣ�
B��KCl�к��м�Ԫ�أ����ڼطʣ�
C��NH4H2PO4�к��е�Ԫ�غ���Ԫ�أ����ڸ��Ϸʣ�
D��KNO3�к��е�Ԫ�غͼ�Ԫ�أ����ڸ��Ϸʣ�
��ѡ��D��
��6������������ࡢ�㳦���������ʡ�ƻ������ά���ء��߸�ţ�̸��������ʣ����ά���أ�
��7��A���ǽ�ֹ���̱�־���ʴ���
B���ǿ�ѭ�����õ�ͼ�꣬����ȷ��
C���ǽ�Լˮ�ı�־���ʴ���
D���ǵ�ͷ��־���ʴ���
��ѡ��B��