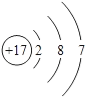
��Ŀ����
��Ҫ��������з���ʽ����д��
��1���и��Ϸ����ɵ��кͷ�Ӧ������ʽ��ʾ��_____________
��2��ijЩ�����⻯��(��������-l��)��ˮ��Ӧ�����ɼ����������NaH+H2O =NaOH+H2��������һ����ѧ����ʽ��ʾCaH2��Na2CO3ˮ��Һ֮�䷢���Ļ�ѧ��Ӧ������ʽ��ʾ��___________
��3��Cu�����ã�ͨ������²���ϡ���ᷴӦ������Cu��ϡ����Ļ�����е���H2O2����Һ�ܿ����ɫ����д���÷�Ӧ�Ļ�ѧ����ʽ ___________________________
��4���������л�ѧ����ʽ��2H2S+SO2=3S��+2H2O������ɻ�ѧ����ʽ����д��NH3��NO2��Ӧ ___________________________________________
Ϊ̽��ij���ϵ����Ԫ�أ������ʵ�� I �� II���ش��������⣺
I����ȡ����
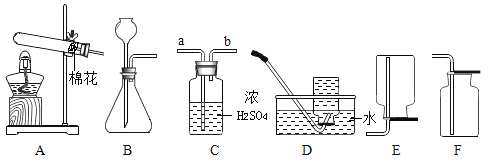
��1������һ�ְ���ɫ������ȡO2��������Ӧ�Ļ�ѧ����ʽΪ______��
��2������ȡ�����O2��װ�ú���������˳��Ϊ������װ��A��__�����ţ�������װ��ʱ������װ�õij�����Ӧ��װ��C��_���a����b������������
II�����̽��
��3�����Լ�⣨��֪����ˮCuSO4��ˮ����ɫ��
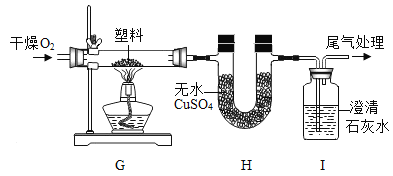
ʵ����� | ʵ������ | ���� |
����װ�ã���������ԣ�װ���Լ�������ͼ����ʵ�顣ͨ��O2��һ��ʱ���ȼ J ���ƾ��ơ� | װ�� K ����ˮCuSO4��_ɫ | ����ȼ�ղ�������H2O |
װ�� L ��_ | ����ȼ�ղ�������CO2 |
��������ʵ���֪��������һ�����е�Ԫ����__����Ԫ�ط��ţ��������ⶨΪ��һ��ȷ����ɣ���1.4g������������O2����ȫȼ�գ���������4.4g CO2��1.8g H2O�����������غ㶨�ɣ����жϸ�����__����� �С������������������н���֮���Ԫ�ء�


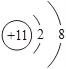 B.
B. C.
C. D.
D.