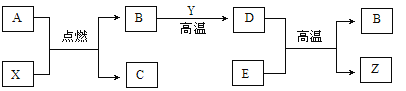
��Ŀ����
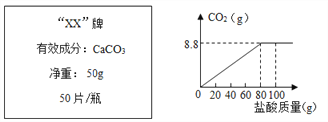
����Ŀ��Ϊ�ⶨij����ҩ���еĺ��������ֽ�100g������μӵ�ʢ����40g��ҩ���Ƴɵķ�ĩ�е��ձ���(�����ɷֲ������ᷴӦ)���õ�����������ͼ�����¡�
������й���Ϣ�ش����⣺
(1)CaCO3�и�Ԫ�ص���������_________��
(2)��Ӧ��ȫʱ����CO2___________��
(3)��������ʵ���ϡ�����Ũ��___________��
(4)ǡ�÷�Ӧ��ȫʱ�����ĵ�̼��Ƶ�����__________���ò��Ƽ��и�Ԫ�ص���������Ϊ___________��
���𰸡� 40�� 8.8g 18.25% 20g 20%
��������������Ҫ�������û�ѧ����ʽ���к�����������ʽ���м������������ͼ��õ���Ӧ��ȫʱ����CO2�������ǽ���Ļ�����
��1��CaCO3�и�Ԫ�ص����������ǣ�![]() ��100%=40%��
��100%=40%��
��2����ͼ���֪����Ӧ��ȫʱ����CO2��������8.8g��
��3���裺ǡ����ȫʱ���������е���������Ϊx���μӷ�Ӧ��̼��Ƶ�����Ϊy��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73 44
y x 8.8g
![]() x=14.6g
x=14.6g
![]() y=20g
y=20g
�����������ʵ���������=![]() ��100%=18.25%��
��100%=18.25%��
��4���ò��Ƽ��и�Ԫ�ص���������=![]() ��100%=20%��
��100%=20%��
����(1)CaCO3�и�Ԫ�ص���������Ϊ40%��
(2)��Ӧ��ȫʱ����CO2������Ϊ8.8g��
(3)��������ʵ���ϡ�����Ũ��Ϊ18.25%��
(4)ǡ�÷�Ӧ��ȫʱ�����ĵ�̼��Ƶ�����Ϊ20g���ò��Ƽ��и�Ԫ�ص���������Ϊ20%��

����Ŀ������ͼ��������ȷ��ʾ��仯���̵��ǣ� ��
|
|
A����pH=10��KOH��Һ�в��ϼ�ˮϡ�� | B����һ��������KClO3��MnO2�Ļ������ȡO2 |
|
|
C����FeCl3��HNO3�Ļ����Һ�м���NaOH��Һֱ������ | D��20��ʱ����һ��������NaCl��Һ�м���KNO3���� |