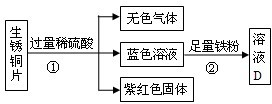
��Ŀ����
��ʽ̼��ͭ�ɱ�ʾΪ��xCuCO3?yCu��OH��2?zH2O��x��y��zȡ���������ⶨ��ʽ̼��ͭ��ɵķ����ж��֣��ֲ���������ԭ������ش��������⣺
��1����ƽxCuCO3?yCu��OH��2?zH2O��������Ӧ�Ļ�ѧ����ʽ
��
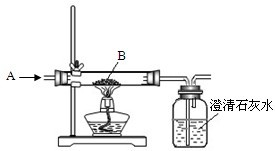
��2��ʵ��װ�������������������Ӷ��ɣ������������������˳����a��k��j��gf��de��hi��bc��l�� �����һ��������м�ʯ�ҵ����ã�

��3����ȡ71.2gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�38.4g���������8.8g������̼��25.2gˮ������Ʒ�Ľᾧˮ����Ϊ
��1����ƽxCuCO3?yCu��OH��2?zH2O��������Ӧ�Ļ�ѧ����ʽ
��
1
1
��xCuCO3?yCu��OH��2?zH2O+��x+y
x+y
��H2=��x+y
x+y
��Cu+��x
x
��CO2��+��x+2y+z
x+2y+z
��H2O��2��ʵ��װ�������������������Ӷ��ɣ������������������˳����a��k��j��gf��de��hi��bc��l�� �����һ��������м�ʯ�ҵ����ã�
��ֹ������CO2��H2O����װ�ã�Ӱ��ⶨ���
��ֹ������CO2��H2O����װ�ã�Ӱ��ⶨ���

��3����ȡ71.2gij��ʽ̼��ͭ��Ʒ����ַ�Ӧ��õ�38.4g���������8.8g������̼��25.2gˮ������Ʒ�Ľᾧˮ����Ϊ
7.2
7.2
g����ѧʽΪCuCO3?2Cu��OH��2?2H2O
CuCO3?2Cu��OH��2?2H2O
����������1�����ݷ�Ӧǰ���ԭ���������������ƽ
��2������ʵ��������ÿ��ʵ��װ�õ����ý��н��
��3�����ݻ�ѧ����ʽ������֮���������ϵ���x��y��z���������ɵ�ˮ�ͷ�Ӧ����ˮ�Ĺ�ϵ���ˮ��������
��2������ʵ��������ÿ��ʵ��װ�õ����ý��н��
��3�����ݻ�ѧ����ʽ������֮���������ϵ���x��y��z���������ɵ�ˮ�ͷ�Ӧ����ˮ�Ĺ�ϵ���ˮ��������
����⣺��1�����ݷ�Ӧǰ��ԭ������������������ƽ������ʽ̼��ͭǰ��ϵ����Ϊ1����Ӧǰͭԭ�Ӹ���Ϊ��x+y�����������ɵ�ͭǰ��ӣ�x+y������Ӧǰ̼ԭ�Ӹ���Ϊx�����������������̼�����x����Ӧǰ��ԭ�Ӹ���Ϊ3x+2y+z�������������̼����ԭ�Ӹ���Ϊ2x��������ԭ�Ӹ�����ȣ�����ˮǰ��ӣ�x+2y+z�������ݷ�Ӧǰ����ԭ�Ӹ�����ȿɵó�����ǰ��ӣ�x+y��
��2������ʵ�����̿��Կ������ø�����������ʽ̼��ͭ��Ӧ��ͨ���ⶨ���ɵĶ�����̼��ˮ�Լ���Ӧ������������ȷ�����ʵ���ɣ�Ϊ��ֹ������CO2��H2O����U����װ�ã��������������һ��ʢ�м�ʯ�ҵĸ���װ�������տ����еĶ�����̼��ˮ��
��3����
xCuCO3?yCu��OH��2?zH2O+�� x+y��H2=�� x+y��Cu+xCO2��+�� x+2y+z��H2O
64��x+y�� 44x 18��x+2y+z��
38.4g 8.8g 25.2g
=
��ã�y=2x
=
��ã�z=2x
��x=1����y=2��z=2
��ѧʽΪCuCO3?2Cu��OH��2?2H2O
��ѧ����ʽΪ
CuCO3?2Cu��OH��2?2H2O+3H2=3Cu+CO2��+7H2O
�ɻ�ѧ����ʽ���Կ�������ʽ̼��ͭÿ����ˮ����������7��ˮ���ӣ������ɵ�ˮ�м�ʽ̼��ͭ��ˮռ��
����˼�ʽ̼��ͭ��ˮ������Ϊ��25.2g��
=7.2g
�ʴ�Ϊ��
��1��1 ��x+y�� ��x+y�� x ��x+2y+z��
��2����ֹ������CO2��H2O����װ�ã�Ӱ��ⶨ ���
��3��7.2
CuCO3?2Cu��OH��2?2H2O ������������Ҳ���֣�
��2������ʵ�����̿��Կ������ø�����������ʽ̼��ͭ��Ӧ��ͨ���ⶨ���ɵĶ�����̼��ˮ�Լ���Ӧ������������ȷ�����ʵ���ɣ�Ϊ��ֹ������CO2��H2O����U����װ�ã��������������һ��ʢ�м�ʯ�ҵĸ���װ�������տ����еĶ�����̼��ˮ��
��3����
xCuCO3?yCu��OH��2?zH2O+�� x+y��H2=�� x+y��Cu+xCO2��+�� x+2y+z��H2O
64��x+y�� 44x 18��x+2y+z��
38.4g 8.8g 25.2g
| 64(x+y) |
| 38.4g |
| 44x |
| 8.8g |
��ã�y=2x
| 44x |
| 8.8g |
| 18(x+2y+z) |
| 25.2g |
��ã�z=2x
��x=1����y=2��z=2
��ѧʽΪCuCO3?2Cu��OH��2?2H2O
��ѧ����ʽΪ
CuCO3?2Cu��OH��2?2H2O+3H2=3Cu+CO2��+7H2O
�ɻ�ѧ����ʽ���Կ�������ʽ̼��ͭÿ����ˮ����������7��ˮ���ӣ������ɵ�ˮ�м�ʽ̼��ͭ��ˮռ��
| 2 |
| 7 |
| 2 |
| 7 |
�ʴ�Ϊ��
��1��1 ��x+y�� ��x+y�� x ��x+2y+z��
��2����ֹ������CO2��H2O����װ�ã�Ӱ��ⶨ ���
��3��7.2
CuCO3?2Cu��OH��2?2H2O ������������Ҳ���֣�
������������һ��Ϣ�⣬�ѶȽϴؼ�����ƽ��ѧ����ʽ���ܸ���ʵ�����̷�����װ�õ����ã��ܸ��ݻ�ѧ����ʽ���м��㣮

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
�����Ŀ
 30���ס��ҡ������������dz��л�ѧ���������ʣ���ͼ�е����Բ�ֱ��ʾ���������ʣ�����Բ���б�ʾ�����������ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ������P��Ӧ��������ȥ����
30���ס��ҡ������������dz��л�ѧ���������ʣ���ͼ�е����Բ�ֱ��ʾ���������ʣ�����Բ���б�ʾ�����������ܷ�����Ӧ����������ʾ���ʼ��ת����ϵ�����ַ�Ӧ������P��Ӧ��������ȥ����