��Ŀ����
�±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
������ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ������������������ǵ��ܶȱ仯���������������Һ�� �����ڰ�ˮ�� ��
��2����100g 24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����Ϊ mL��
��3������������Ϊ24%�������м���������ˮ��������Һ�������������� 12%������������Ϊ24%�İ�ˮ�м���������ˮ��������Һ�������������� 12%���á�������������=����գ����������ϼ���������Ľ�����ܽ���Ĺ����� ��
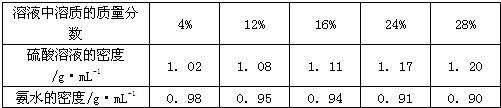
| ��Һ�����ʵ� �������� |
4% | 12% | 16% | 24% | 28% |
| ������Һ�� �ܶ�/g?mL-1 |
1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g?mL-1 | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ������������������ǵ��ܶȱ仯���������������Һ��
��2����100g 24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����Ϊ
��3������������Ϊ24%�������м���������ˮ��������Һ��������������
��������1�����ݱ������ݺ���������������Һ���ܶ����������������仯�Ĺ��ɣ�
��2�����ݷ���֮���м����������Ϊ��Ϻ�������Һ������������ߵ����֮�ͽ��н��
��3������һ������������������Һ�͵������ˮ��ϣ�������Һ�����ʵ�����������������������������Һ�ܶȵĵݱ���ɵ�Ӱ����н��
��2�����ݷ���֮���м����������Ϊ��Ϻ�������Һ������������ߵ����֮�ͽ��н��
��3������һ������������������Һ�͵������ˮ��ϣ�������Һ�����ʵ�����������������������������Һ�ܶȵĵݱ���ɵ�Ӱ����н��
����⣺��1�����ݱ������ݺ���������������Һ���ܶ����������������仯�Ĺ��ɣ�����������Һ��������������Խ���ܶ�Խ���ڰ�ˮ��������������Խ���ܶ�ԽС��
��2�����ڷ���֮���м����������Ϊ��Ϻ�������Һ������������ߵ����֮�ͣ�������Һ�����ʵ���������Ϊ
��100%=12%������Һ���ܶ�Ϊ0.95 g?mL-1��������Ϊ
��210.5 mL��
��3����ԭ��Һ������Ϊm1������ˮ������Ϊm2������������Һ���ܶȱ�ˮ�������������ȣ����m1��m2���ʻ�Ϻ���Һ�����ʵ���������Ϊ
��24%��
��24%=12%�����ڰ�ˮ���ܶȱ�ˮС�������������ȣ����m1��m2���ʻ�Ϻ���Һ�����ʵ���������Ϊ
��24%��
��24%=12%��ͨ�������֪��һ������������������Һ�͵������ˮ��ϣ�������Һ�����ʵ�����������������������������Һ�ܶȵĵݱ���ɵ�Ӱ�죮
�𰸣���1��������������Խ���ܶ�Խ��������������Խ���ܶ�ԽС��
��2��210.5��
��3������������������������Խ���ܶ�Խ������ʣ�����Һ�͵������ˮ��ϣ�������Һ���������������������������������Ķ���֮һ����������������Խ���ܶ�ԽС�����ʣ�����Һ�͵������ˮ��ϣ�������Һ��������������С�����������������Ķ���֮һ��
��2�����ڷ���֮���м����������Ϊ��Ϻ�������Һ������������ߵ����֮�ͣ�������Һ�����ʵ���������Ϊ
| 100g��24% |
| 100g+100g |
| 100g+100g |
| 0.95g?mL-1 |
��3����ԭ��Һ������Ϊm1������ˮ������Ϊm2������������Һ���ܶȱ�ˮ�������������ȣ����m1��m2���ʻ�Ϻ���Һ�����ʵ���������Ϊ
| m1 |
| m1+m2 |
| m1 |
| 2m1 |
| m1 |
| m1+m2 |
| m1 |
| 2m1 |
�𰸣���1��������������Խ���ܶ�Խ��������������Խ���ܶ�ԽС��
��2��210.5��
��3������������������������Խ���ܶ�Խ������ʣ�����Һ�͵������ˮ��ϣ�������Һ���������������������������������Ķ���֮һ����������������Խ���ܶ�ԽС�����ʣ�����Һ�͵������ˮ��ϣ�������Һ��������������С�����������������Ķ���֮һ��
������������Ҫ������������ʵ����������뻯ѧ���ʵ���Է������йص���Ŀ���Ѷ�һ�㣬�ǻ����⣻�����Ҫ���������Һ�ܶ�����Һ��������֮��ı仯��ϵ�������ܹ�����Է��������ĽǶȽ��з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
��Һ�����������ϢϢ��أ���Һ���������ճ�����ͻ�ѧʵ���еij����������±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
����ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ��� �����������䣩����ˮ���ܶ��� ��������С�䣩
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ 100g������ڡ�С�ڻ���ڣ���
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ����� mL��������0.1����
| ��Һ�����ʵ���������/% | 4 | 12 | 16 | 24 | 28 |
| ������Һ���ܶ�/g/mL | 1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g/mL | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ���
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����
��Һ�����������ϢϢ��أ���Һ���������ճ�����ͻ�ѧʵ���еij����������±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
����ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ��� �����������䣩����ˮ���ܶ��� ��������С�䣩
��2��ȡ12%��������Һ100%���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������Ӧ 100g������ڡ�С�ڻ���ڣ���
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ����� mL��������0.1����
| ��Һ�����ʵ���������/% | 4 | 12 | 16 | 24 | 28 |
| ������Һ���ܶ�/g/mL | 1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g/mL | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ���
��2��ȡ12%��������Һ100%���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������Ӧ
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����