��Ŀ����
����Ŀ���ҹ�ú̿��Դ�ḻ��ú������ȼ���⣬������Ҫ�Ļ���ԭ�ϡ���ҵ����ú�Ϳ���Ϊԭ����������[CO��NH2��2]��һ���������£�
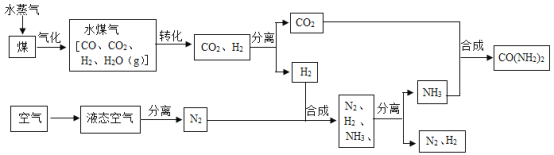
��1����Һ̬�����з����N2�Ĺ�������_____�����������ѧ�����仯��
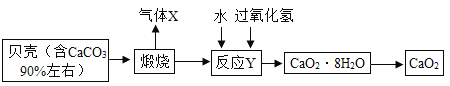
��2����ú��ˮ������Ӧǰ���Ƚ�ú���飬��������Ŀ����_____��
��3�����������з�������Ҫ��ӦΪ��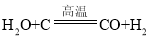 ��
��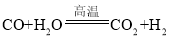 �����������û���Ӧ����_____����١��ڡ�������Ӧ������Ԫ�صĻ��ϼ۱仯Ϊ+1��0��̼Ԫ�صĻ��ϼ۱仯Ϊ_____����Ӧ���з�����ԭ��Ӧ��������_____��д��ѧʽ����
�����������û���Ӧ����_____����١��ڡ�������Ӧ������Ԫ�صĻ��ϼ۱仯Ϊ+1��0��̼Ԫ�صĻ��ϼ۱仯Ϊ_____����Ӧ���з�����ԭ��Ӧ��������_____��д��ѧʽ����
��4���ڸ��¸�ѹ�£�CO2��NH3���Ժϳ����أ�ͬʱ����ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��_____��
��5��ʵ�������У�N2��H2������ȫ��ת��ΪNH3�����������п���ѭ�����õ������У�_____��
���𰸡����� ����ú����ˮ�����ĽӴ������ʹ��Ӧ����� �� 0��+2 H2O CO2��2NH3![]() CO(NH2)2��H2O ����������
CO(NH2)2��H2O ����������
��������
��1����Һ̬�����н��¡���ѹ�����N2�Ĺ�������û�������ʲ��������������仯��
��2����ú��ˮ������Ӧǰ���Ƚ�ú���飬��������Ŀ���ǣ�����ú����ˮ�����ĽӴ������ʹ��Ӧ����֣�
��3�����������з�������Ҫ��ӦΪ�� ��
��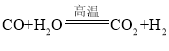 �����������û���Ӧ���Ǣ٣����ϡ�����+������=�µ���+�»��������Ӧ������Ԫ�صĻ��ϼ۱仯Ϊ+1��0��̼Ԫ�صĻ��ϼ۱仯Ϊ0��+2����Ӧ����ˮ�е�������ȥ��������������������ԭ��Ӧ��������ˮ��H2O��
�����������û���Ӧ���Ǣ٣����ϡ�����+������=�µ���+�»��������Ӧ������Ԫ�صĻ��ϼ۱仯Ϊ+1��0��̼Ԫ�صĻ��ϼ۱仯Ϊ0��+2����Ӧ����ˮ�е�������ȥ��������������������ԭ��Ӧ��������ˮ��H2O��
��4���ڸ��¸�ѹ�£�CO2��NH3���Ժϳ����أ�ͬʱ����ˮ���ɵĻ�ѧ����ʽΪ��CO2��2NH3![]() CO(NH2)2��H2O��
CO(NH2)2��H2O��
��5��ʵ�������У�N2��H2������ȫ��ת��ΪNH3��ÿ�κϳɰ�ʱ������ʣ�࣬�������������Ǻϳɰ���ԭ�ϣ����������п���ѭ�����õ������У�������������