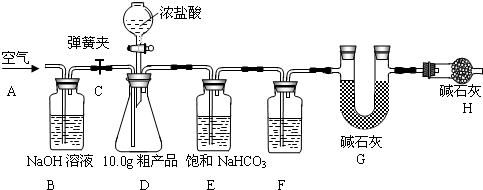
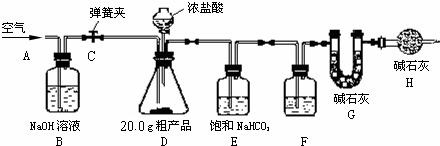
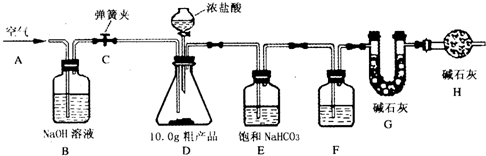
��Ŀ����
ij������ȤС�����÷�ͭм��ȡCuSO4����Ʒ������£��ף�Cu
| ||
| �� |
| ||
| �� |
�ң�Cu
| ||
| �� |
| ||
����Cu
| AgNO3 |
| ||
| ��Һ |
| ϡH2SO4 |
����Cu
| FeSO4 |
�����ۡ����������á������ȷ��濼�ǣ���ÿ���������м�Ҫ���ۣ�������ȷ��һ�����ŷ�����
������
�ҷ�����
��������
��������
��������ÿһ������Ҫ�����ۣ����ã������������ȷ��濼�ǣ�
���������ۡ����á������������ȷ��涼�ܺã�
�ҷ��������ɶ��������ж����壬��������
�������������ӣ������ã�
��������Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
���������ۡ����á������������ȷ��涼�ܺã�
�ҷ��������ɶ��������ж����壬��������
�������������ӣ������ã�
��������Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
����⣺������Cu�ڿ��������գ�����������������ͭ������ͭ����ϡ���ᷴӦ��������ͭ�������ۡ����á������������ȷ��涼�ܺã�
�ҷ�����Cu��Ũ���ᷴӦ�����ɶ��������ж����壬����Ⱦ��������������
���������������ӣ���Ҫ�õ��������ȹ����Լ������Ǻõķ�����
��������Cu������������Һ��������Ӧ����Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
�ʴ�Ϊ��
Cu�ڿ��������գ�����������������ͭ������ͭ����ϡ���ᷴӦ��������ͭ�������ۡ����á������������ȷ��涼�ܺã�
Cu��Ũ���ᷴӦ�����ɶ��������ж����壬����Ⱦ��������������
�������ӣ���Ҫ�õ��������ȹ����Լ������Ǻõķ�����
Cu������������Һ��������Ӧ����Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
�ҷ�����Cu��Ũ���ᷴӦ�����ɶ��������ж����壬����Ⱦ��������������
���������������ӣ���Ҫ�õ��������ȹ����Լ������Ǻõķ�����
��������Cu������������Һ��������Ӧ����Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
�ʴ�Ϊ��
Cu�ڿ��������գ�����������������ͭ������ͭ����ϡ���ᷴӦ��������ͭ�������ۡ����á������������ȷ��涼�ܺã�
Cu��Ũ���ᷴӦ�����ɶ��������ж����壬����Ⱦ��������������
�������ӣ���Ҫ�õ��������ȹ����Լ������Ǻõķ�����
Cu������������Һ��������Ӧ����Ϊͭ�Ļ�Ա������������û����������������в�ͨ��
����������ͬѧ�ǴӶ�Ƕ�˼���������������ȡһ�����ʿ����кܶ����Ҫѡ�����ŷ����������������Ҳ��һ����

��ϰ��ϵ�д�
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ