��Ŀ����
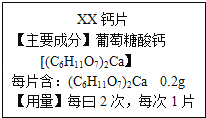
����Ŀ��ˮ����Ҫ�ɷ���̼��ơ�������þ������ˮ��ʵ��������һˮ����Ʒ��Ϊ�ⶨ���и��ɷݵ�����������ij��ȤС���ͬѧ������ͼ��ʾװ�ý���ʵ�飨��װ�����������ã�A��C��D����װҩƷ��������
��֪���ټ�ʯ���������ƺ��������ƵĻ���
�ڸ��������£�̼��ơ�������þ���ֽ�Ϊ���������
����ʵ�鲽�����£�
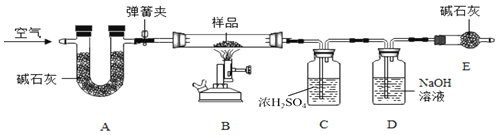
��ȡ8g��Ʒװ��װ��B�IJ������У���ͼ���Ӻ�װ�ã��رյ��ɼУ�����Ʒ���ȣ�
����Ʒ��ȫ��Ӧ��______������ĸ��ţ���
A �ȴ��ɼ�ͨ���������Ϩ��ƾ���ƣ�ֱ����ȴ��
B ��Ϩ��ƾ���ƣ���������ȴ���ٴ��ɼ�ͨ�����
��ʵ����ϣ����װ��C��D�е�Һ�������ֱ�������1g��2.2g��
��������ʵ���������ݼ������Ʒ��̼��ơ�������þ��������������ش�
��1��д��������þ�ֽ�ķ�Ӧ����ʽΪ______��
��2����Ӧ��������������Ŀ����______��
��3��װ��B�еķ�Ӧ����ʽ______��______��
��4��װ��C��������______��D��������______��
��5�����ˮ����Ʒ��̼��Ƶ�����������______��д��������̣�
���𰸡�A Mg��OH��2 ![]() MgO+H2O ʹ��Ӧ���ɵ�ˮ������������̼ȫ����B��Cװ������ CaCO3
MgO+H2O ʹ��Ӧ���ɵ�ˮ������������̼ȫ����B��Cװ������ CaCO3![]() CaO+CO2���� Mg��OH��2
CaO+CO2���� Mg��OH��2![]() MgO+H2O ����ˮ���� ���ն�����̼ 62.5%
MgO+H2O ����ˮ���� ���ն�����̼ 62.5%
��������
����Ʒ��ȫ��Ӧ���ȴ��ɼ�ͨ���������Ϩ��ƾ���ƣ�ֱ����ȴ��������Ϩ��ƾ���ƣ��Է�ֹҺ�嵹��ը�Ѳ����ܡ�
���A��
������1������������������þ�ֽ���������þ��ˮ����Ӧ�ķ�Ӧ����ʽΪ��Mg��OH��2![]() MgO+H2O�����Mg��OH��2
MgO+H2O�����Mg��OH��2![]() MgO+H2O��
MgO+H2O��
��2����Ӧ��������������Ŀ����ʹ��Ӧ���ɵ�ˮ������������̼ȫ����B��Cװ�����ա�
���ʹ��Ӧ���ɵ�ˮ������������̼ȫ����B��Cװ�����ա�
��3��װ��B�У�����������̼��Ʒֽ����������ƺͶ�����̼��������þ�ֽ���������þ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3![]() CaO+CO2����Mg��OH��2
CaO+CO2����Mg��OH��2![]() MgO+H2O��
MgO+H2O��
���CaCO3![]() CaO+CO2����Mg��OH��2
CaO+CO2����Mg��OH��2![]() MgO+H2O��
MgO+H2O��
��4��װ��C������������ˮ������D�����������ն�����̼��
�������ˮ���������ն�����̼��
��5����̼�������Ϊx��

![]()
x=5g��
��ˮ����Ʒ��̼��Ƶ���������Ϊ��![]() ��100%=62.5%��
��100%=62.5%��
�𣺸�ˮ����Ʒ��̼��Ƶ���������Ϊ62.5%��

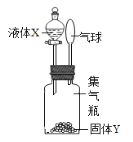
����Ŀ����ͼ��ʾ����Һ��X���뵽����ƿ�������Y���ã��۲쵽������������Һ��X����Y����ϣ�����������ǣ�������
�� | �� | �� | �� | �� | |
X | ϡ���� | ˮ | ˮ | ˫��ˮ | ˮ |
Y | ���� | �������� | �Ȼ��� | �������� | ����� |
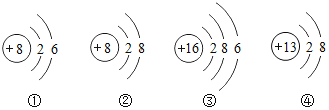
A. �٢ڢ� B. �٢ۢ� C. �٢ڢ� D. �ڢۢ�
����Ŀ��ij��ѧ�С�����ѧϰ�������ƺ��������Ƶ����֪ʶ�������Ca��OH��2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
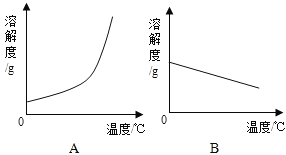
��1�������ϱ����ݣ�������Ca��OH��2��NaOH���ܽ�����ߣ�ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_____���A����B������
��2����һƿ�ӽ����͵�Ca��OH��2��Һ��ɱ�����Һ�������ʩ�У�
�������������������������¶��������¶�������ˮ������ˮ���ٻָ���ԭ�¶�������������ʯ�ҡ�
���д�ʩ��ԭ������ȷ����_____������ĸ��
A �ڢܢ� B �ۢ� C �٢ڢݢ� D �٢ڢ�
��3��20��ʱ��191g����NaOH��Һ������10gˮ���ٻָ���20�棬������NaOH���塣��ʱ��Һ����������Ϊ_____
��4��20��ʱ�����ⶨNaOH��Һ��pH�����Ƚ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pH_____���ƫ��ƫС��������Ӱ�족����
��5���ɼ�������������Һ�ͳ���ʯ��ˮ��һ����ѧ��Ӧ����ʽΪ_____��