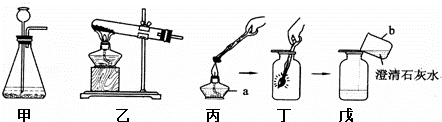
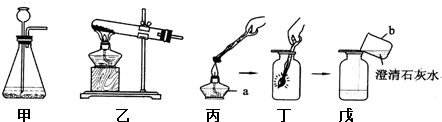
��Ŀ����
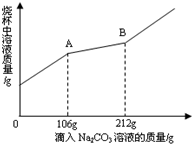 ij��ѧ����С����ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼�������ʵ��Է�Ӧ��ķ�Һ����̽��������ȡ���˺�ķ�Һ100g���������μ����ʵ���������Ϊ10%��Na2CO3��Һ���ձ�����Һ��������������Na2CO3��Һ��������ϵ��ͼ��ʾ��
ij��ѧ����С����ʯ��ʯ��ϡ����Ϊԭ����ȡ������̼�������ʵ��Է�Ӧ��ķ�Һ����̽��������ȡ���˺�ķ�Һ100g���������μ����ʵ���������Ϊ10%��Na2CO3��Һ���ձ�����Һ��������������Na2CO3��Һ��������ϵ��ͼ��ʾ�����������ش��������⣺
��1����ʵ������У�������ų��������Կ���������ʵ��������
������ɫ����
������ɫ����
���ɴ˵ó����˺�ķ�Һ�к��е�����ΪCaCl2 ��HCl
CaCl2 ��HCl
�����ѧʽ����2����ʵ������зų��������������
4.4
4.4
g����3��������Na2CO3��Һ��ͼ��B��ʱ�����ʱ���ò�������Һ�����ʵ�������
��������1����ȡ������̼��Ӧ��ķ�Һ���μ�Na2CO3��Һ��������ų���˵����Һ����HCl����Na2CO3��Һ����ʱ��̼���ƺ��Ȼ��Ʒ�Ӧ����̼�������ɫ���������н��
��2������ͼʾ���ݣ�10%��Na2CO3��Һ����������ų��������������
��3������ͼʾ���ݣ��ֱ�����A�� Na2CO3 ��HCl�����Ȼ��Ƶ��ʡ�B��Na2CO3 �� CaCl2 �����Ȼ��Ƶ����������ɽ��
��2������ͼʾ���ݣ�10%��Na2CO3��Һ����������ų��������������
��3������ͼʾ���ݣ��ֱ�����A�� Na2CO3 ��HCl�����Ȼ��Ƶ��ʡ�B��Na2CO3 �� CaCl2 �����Ȼ��Ƶ����������ɽ��
����⣺��1����ȡ������̼��Ӧ��ķ�Һ���μ�Na2CO3��Һ��������ų���˵����Һ����HCl����Na2CO3��Һ����ʱ��̼���ƺ��Ȼ��Ʒ�Ӧ����̼�������ɫ�������ɴ˿�֪��Һ�к��е�����Ϊ��CaCl2��HCl��
�ʴ�Ϊ��������ɫ������CaCl2��HCl��
��2����ʵ������зų������������Ϊa
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
106g��10% a
=
��� a=4.4g
�ʴ�Ϊ��4.4g��
��3���⣺106g10%��̼������Һ�к� Na2CO3 �������ǣ�106g��10%=10.6g
�跴Ӧ������ A��ʱ���ɵ��Ȼ��Ƶ�����Ϊ x��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117
10.6g x
=
x=11.7g
�� A������� B ��μ�̼���Ƶ���������212g-106g����10%=10.6g
�跴Ӧ�� A ������� B ��ʱ���ɵ��Ȼ��Ƶ�����Ϊ y��
Na2CO3+CaCl2=2NaCl+CaCO3��
106 117
10.6g y
=
y=11.7g
�ձ��ﲻ������Һ�����ʵ�����Ϊ��11.7g+11.7g=23.4g
���ձ��ﲻ������Һ�����ʵ�����Ϊ 23.4g��
�ʴ�Ϊ��������ɫ������CaCl2��HCl��
��2����ʵ������зų������������Ϊa
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
106g��10% a
| 106 |
| 44 |
| 1.6g��10% |
| a |
�ʴ�Ϊ��4.4g��
��3���⣺106g10%��̼������Һ�к� Na2CO3 �������ǣ�106g��10%=10.6g
�跴Ӧ������ A��ʱ���ɵ��Ȼ��Ƶ�����Ϊ x��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 117
10.6g x
| 106 |
| 117 |
| 10.6g |
| x |
�� A������� B ��μ�̼���Ƶ���������212g-106g����10%=10.6g
�跴Ӧ�� A ������� B ��ʱ���ɵ��Ȼ��Ƶ�����Ϊ y��
Na2CO3+CaCl2=2NaCl+CaCO3��
106 117
10.6g y
| 106 |
| 117 |
| 10.6g |
| y |
�ձ��ﲻ������Һ�����ʵ�����Ϊ��11.7g+11.7g=23.4g
���ձ��ﲻ������Һ�����ʵ�����Ϊ 23.4g��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
�����Ŀ
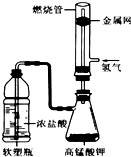 ij��ѧ����С������ͼ��ʾ��װ����ȡ������Cl2����ģ�����Ṥҵ�ϳ��Ȼ��⣨���������Է�ֹ������ȼ�չ����з�����ը����
ij��ѧ����С������ͼ��ʾ��װ����ȡ������Cl2����ģ�����Ṥҵ�ϳ��Ȼ��⣨���������Է�ֹ������ȼ�չ����з�����ը����