��Ŀ����
ijͬѧ����ͼ��ʾ��װ�����ⶨ���������������ĺ�����ʵ�飮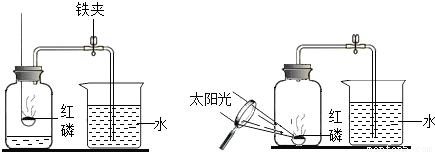
��1�������ڼ���ƿ��ȼ�գ��ɹ۲쵽��������______��
��2������Ϩ����ȴ���ɼк۲쵽��������______��
��3��ȼ�պ���ƿ�ڵ���Ҫ������Ҫ�ǵ��������ϱ�ʵ���Ʋ�������ʣ�
�������ʣ�______��
��ѧ���ʣ�______��
��4��ʵ���з��ֽ��뼯��ƿ�е�ˮδ�ﵽԤ���ĸ߶ȣ������ԭ��______��
��5��ʵ�������С��ͬѧ��ʵ������˷�˼������˸Ľ��ķ�������ͼ��ʾ��������Ϊ�����Ľ����ŵ��У�______��
���𰸡���������1�����ݺ���ȼ��ʱ������������
��2�����ݺ���ȼ�պ������˼���ƿ�е����������¼���ƿ�ڵ�ѹǿ��С�������
��3������ʵ����ɺ������ʣ�࣬��ƿ��ʣ���������ҪΪ�������ɴ˿��ƶϵõ���������ȼ��Ҳ��֧��ȼ�յĻ�ѧ���ʣ���������ˮ���棬˵��������������ˮ��
��4��ʵ����ϣ������뼯��ƿ��ˮ������������ݻ��� ��˵��ƿ���������������
��˵��ƿ��������������� ��������ԭ����ҪΪ����δ��ȫ���Ļ�����ȴ�����п�������ƿ�������µĽ�����ݴ˶�ʵ����з�����
��������ԭ����ҪΪ����δ��ȫ���Ļ�����ȴ�����п�������ƿ�������µĽ�����ݴ˶�ʵ����з�����
��5���ԱȸĽ���ǰ�������̽��װ�ã��ɷ��ָĽ�����Ҫ��ȼ�����ٽ�ȼ�ճ�����ƿ�У��ɺܺõر�����װ��©�����Խ��������ƫ�
����⣺��1������ȼ��ʱ�����ͬʱ�����������̣�
��2�����ݺ���ȼ�պ������˼���ƿ�е����������¼���ƿ�ڵ�ѹǿ��С��֪�����ɼк۲쵽��������ˮ���뼯��ƿ��
��3����������ȼ�ջ���֧��ȼ�գ�������ʣ�����岻���ǵ�����ʣ�൪����������С����ʵ����ɺ�ʣ����������е�������Ҳû�м��٣�˵�����ײ����ڵ����м���ȼ�գ����жϵ�������֧��ȼ��Ҳ����ȼ�գ�����ʵ���ˮ���뼯��ƿ��ʣ������干�棬���Կ�֪������������ˮ��
��4�����ʵ��������������������û����ȫ���Ļ���װ��©��������ȴ�����н�������������δ��ȴ�����¶����ɼеȣ������ܳ��ֽ��뼯��ƿ��ˮ������������ݻ��� �Ľ����
�Ľ����
��5���Ľ����װ��ֱ��ͨ����̫�������ȼ�ף��������ٴδ�ƿ��������װ��ʼ���ܷ⣬�����˲�������Ϊ��������ɵ�ƫ��ɵõ���ȷ��ʵ������
�ʴ�Ϊ��
��1������ȼ�����ɴ������̣��ų�������
��2��ˮ���뵽����ƿ�У�
��3����������ˮ������ȼ�գ�Ҳ��֧��ȼ�գ�
��4��װ��©���������������ȣ���
��5��װ��ʼ���ܱգ�����û���ݳ���ʵ����ȷ��
����������ʵ�����ͼ������ʵ����ע������Ӷ��ش��ʵ����й����⣮����ʵ��ĸĽ���Ҫ��ԭ���������Dz����к��������ۣ�
��2�����ݺ���ȼ�պ������˼���ƿ�е����������¼���ƿ�ڵ�ѹǿ��С�������
��3������ʵ����ɺ������ʣ�࣬��ƿ��ʣ���������ҪΪ�������ɴ˿��ƶϵõ���������ȼ��Ҳ��֧��ȼ�յĻ�ѧ���ʣ���������ˮ���棬˵��������������ˮ��
��4��ʵ����ϣ������뼯��ƿ��ˮ������������ݻ���
 ��˵��ƿ���������������
��˵��ƿ��������������� ��������ԭ����ҪΪ����δ��ȫ���Ļ�����ȴ�����п�������ƿ�������µĽ�����ݴ˶�ʵ����з�����
��������ԭ����ҪΪ����δ��ȫ���Ļ�����ȴ�����п�������ƿ�������µĽ�����ݴ˶�ʵ����з�������5���ԱȸĽ���ǰ�������̽��װ�ã��ɷ��ָĽ�����Ҫ��ȼ�����ٽ�ȼ�ճ�����ƿ�У��ɺܺõر�����װ��©�����Խ��������ƫ�
����⣺��1������ȼ��ʱ�����ͬʱ�����������̣�
��2�����ݺ���ȼ�պ������˼���ƿ�е����������¼���ƿ�ڵ�ѹǿ��С��֪�����ɼк۲쵽��������ˮ���뼯��ƿ��
��3����������ȼ�ջ���֧��ȼ�գ�������ʣ�����岻���ǵ�����ʣ�൪����������С����ʵ����ɺ�ʣ����������е�������Ҳû�м��٣�˵�����ײ����ڵ����м���ȼ�գ����жϵ�������֧��ȼ��Ҳ����ȼ�գ�����ʵ���ˮ���뼯��ƿ��ʣ������干�棬���Կ�֪������������ˮ��
��4�����ʵ��������������������û����ȫ���Ļ���װ��©��������ȴ�����н�������������δ��ȴ�����¶����ɼеȣ������ܳ��ֽ��뼯��ƿ��ˮ������������ݻ���
 �Ľ����
�Ľ������5���Ľ����װ��ֱ��ͨ����̫�������ȼ�ף��������ٴδ�ƿ��������װ��ʼ���ܷ⣬�����˲�������Ϊ��������ɵ�ƫ��ɵõ���ȷ��ʵ������
�ʴ�Ϊ��
��1������ȼ�����ɴ������̣��ų�������
��2��ˮ���뵽����ƿ�У�
��3����������ˮ������ȼ�գ�Ҳ��֧��ȼ�գ�
��4��װ��©���������������ȣ���
��5��װ��ʼ���ܱգ�����û���ݳ���ʵ����ȷ��
����������ʵ�����ͼ������ʵ����ע������Ӷ��ش��ʵ����й����⣮����ʵ��ĸĽ���Ҫ��ԭ���������Dz����к��������ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 20��ij��ɫ������ֻ����̼��������Ԫ�أ�һ��ͬѧ����ͼ��ʾ��ʵ����̽��������ijɷ֣�ʵ���й۲쵽��ɫ������ɺ�ɫ�������ʯ��ˮ����ǣ�
20��ij��ɫ������ֻ����̼��������Ԫ�أ�һ��ͬѧ����ͼ��ʾ��ʵ����̽��������ijɷ֣�ʵ���й۲쵽��ɫ������ɺ�ɫ�������ʯ��ˮ����ǣ� ��2012?�ߴ���һģ��С��ͬѧ����ͼ��ʾװ�òⶨij������Ʒ������ΪNaCl����Na2CO3������������CΪ�п̶ȵIJ�����������װ���ʵ�Һ�壬����Һ��λ�ñ仯�ⶨ���������
��2012?�ߴ���һģ��С��ͬѧ����ͼ��ʾװ�òⶨij������Ʒ������ΪNaCl����Na2CO3������������CΪ�п̶ȵIJ�����������װ���ʵ�Һ�壬����Һ��λ�ñ仯�ⶨ���������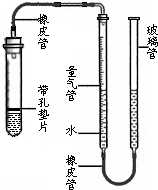 ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ� ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�