��Ŀ����
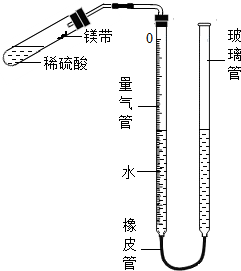
 ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
��ȡһ��ʯ��ʯ��Ʒ��ȷ�Ƶ�������Ϊ1.003g��
������������װˮ�����ڿ̶ȡ�0����λ�ã���ͼ��ʾ��δװҩƷ��װ���������
�ۼ��װ�������ԣ�
�����Թ��м���һ������ϡ���ᣬҺ���Ե��ڵ�Ƭ��
��С�ķ���ʯ��ʯ��Ʒ�����ڵ�Ƭ�ϣ�������Ƥ����
����������Һ�棬ʹ���ߵ�Һ�汣��ͬһˮƽ����¼��������Һ��λ��
�߽���ij������ʹʯ��ʯ��ϡ����Ӵ���Ӧ��
�����ȫ��Ӧ���ٴε�����������Һ�棬����¼Һ��λ�ã�
����������������ӵ��������Ϊ190mL
��֪��ͬ��ͬѹ�£���ͬ�����Ϻ�������ڻ��ǰ���������֮�ͣ�
��ش��������⣺
��1��д��ʯ��ʯ��ϡ���ᷴӦ�Ļ�ѧ����ʽ________��
��2���ܷ���������ƽ����1.003gʯ��ʯ��Ʒ________������ܡ����ܡ���
��3����ʵ����̢��У����кβ�������ʹʯ��ʯ��ϡ����Ӵ���Ӧ��________��
��4����ʵ����̢��У�ͨ��________����˵����Ӧ�Ѿ�ֹͣ��
��5�������㣬��ʵ���õĶ�����̼����ԼΪ0.374g�����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ________��
��6��ͬѧ��ʵ����������۷��֣����βⶨ��̼��ƺ������ܻ�ƫС��ԭ������ǣ�________��________��
�⣺��1��̼��ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ��ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O
��2��������ƽֻ�ܳ���0.1g���ʴ�Ϊ������
��3�����Թ���б����ʹʯ��ʯ��ϡ����Ӵ���Ӧ���ʴ�Ϊ�����Թ���б
��4�����û������ð������֤����Ӧ�����ʴ�Ϊ��û������ð��
��5����̼��Ƶ���������Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
1.003gx 0.374g
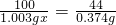 x=85%
x=85%
�ʴ�Ϊ��85%
��6��������̼������ˮ��һ���ֶ�����̼����ˮ���ˣ������ֵƫС���ʴ�Ϊ�������Ķ�����̼����ֵƫС����������̼����ˮ��һ���֣����Թ������ж�����̼���ڣ�
������ʵ������ȡCO2����̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼��������ƽֻ�ܳ���0.1g����ʵ������У����Թ���б����ʹʯ��ʯ��ϡ����Ӵ���Ӧ�����û������ð������֤����Ӧ���������ݻ�ѧ����ʽ�ļ��㣬������������������̼������ˮ��һ���ֶ�����̼����ˮ���ˣ�
�������������м���������ƽ��ʹ�ú�ʵ�����ݵĴ��������л�ѧ����ʽ����д��ʵ�鷽�������ۣ�����ıȽ�ȫ�棬ֻҪ�������������Ͳ��ѽ����ʵ��̽���е����ݵĴ������йصļ������п����ȵ����ݣ�����ȿ�����ʵ���������������չ��Ӧ�У��ֿ�����ѧ���ļ����������ۺ��ԱȽ�ǿ������������ѡ���⡢������ʵ�����У�ͬѧ��Ҫ�������գ�
��2��������ƽֻ�ܳ���0.1g���ʴ�Ϊ������
��3�����Թ���б����ʹʯ��ʯ��ϡ����Ӵ���Ӧ���ʴ�Ϊ�����Թ���б
��4�����û������ð������֤����Ӧ�����ʴ�Ϊ��û������ð��
��5����̼��Ƶ���������Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
1.003gx 0.374g
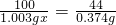 x=85%
x=85%�ʴ�Ϊ��85%
��6��������̼������ˮ��һ���ֶ�����̼����ˮ���ˣ������ֵƫС���ʴ�Ϊ�������Ķ�����̼����ֵƫС����������̼����ˮ��һ���֣����Թ������ж�����̼���ڣ�
������ʵ������ȡCO2����̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼��������ƽֻ�ܳ���0.1g����ʵ������У����Թ���б����ʹʯ��ʯ��ϡ����Ӵ���Ӧ�����û������ð������֤����Ӧ���������ݻ�ѧ����ʽ�ļ��㣬������������������̼������ˮ��һ���ֶ�����̼����ˮ���ˣ�
�������������м���������ƽ��ʹ�ú�ʵ�����ݵĴ��������л�ѧ����ʽ����д��ʵ�鷽�������ۣ�����ıȽ�ȫ�棬ֻҪ�������������Ͳ��ѽ����ʵ��̽���е����ݵĴ������йصļ������п����ȵ����ݣ�����ȿ�����ʵ���������������չ��Ӧ�У��ֿ�����ѧ���ļ����������ۺ��ԱȽ�ǿ������������ѡ���⡢������ʵ�����У�ͬѧ��Ҫ�������գ�

��ϰ��ϵ�д�
�����Ŀ
 ij����С��ͬѧ��ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ��ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ� ij����С��ͬѧ����ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�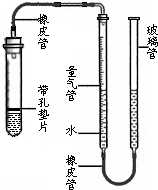 ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ����ͼ��ʾ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�ʯ��ʯ��Ʒ��̼��Ƶ�������������������Ӵ����������壩��ʵ������ǣ� ij����С��ͬѧ��ͼ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ��ͼ��װ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�