��Ŀ����
 ����ͼ��ʾ���ܱ���������һ������������������磬���ܷ��Ծ��Կɿ������������������
����ͼ��ʾ���ܱ���������һ������������������磬���ܷ��Ծ��Կɿ������������������| 1 |
| 4 |
| 1 |
| 4 |
| 3 |
| 4 |
| 1 |
| 2 |
�������������֪����������ͣ�����������
������֪��Ӧ��ʣ���������ԭ�ȿ��������һ�£��������������Ļ��������ԭ����ռ�ܱ�������
����Ӧ��ʣ��
������Ӧ�˻�������
��
| 1 |
| 2 |
| 3 |
| 4 |
| 1 |
| 4 |
| 2 |
| 3 |
����⣺����������������Ӧ�Ļ�ѧ����ʽ��2H2+O2
2H2O��֪
A�������������������Ϊ8��1ʱ��������ȫ��Ӧ������2�����������ʣ������������6����Ӧ��ԭ��������
����A���������⣻
B�������������������Ϊ7��2ʱ��������ȫ��Ӧ������4�����������ʣ������������3����Ӧ��ԭ��������
����B�������⣻
C�������������������Ϊ4��5ʱ����������ȫ��Ӧ������2�����������ʣ������������3����Ӧ��ԭ��������
����C�������⣻
D�������������������Ϊ2��7ʱ����������ȫ��Ӧ������1�����������ʣ������������6����Ӧ��ԭ��������
����D���������⣻
��ѡBC
| ||
A�������������������Ϊ8��1ʱ��������ȫ��Ӧ������2�����������ʣ������������6����Ӧ��ԭ��������
| 1 |
| 3 |
B�������������������Ϊ7��2ʱ��������ȫ��Ӧ������4�����������ʣ������������3����Ӧ��ԭ��������
| 2 |
| 3 |
C�������������������Ϊ4��5ʱ����������ȫ��Ӧ������2�����������ʣ������������3����Ӧ��ԭ��������
| 2 |
| 3 |
D�������������������Ϊ2��7ʱ����������ȫ��Ӧ������1�����������ʣ������������6����Ӧ��ԭ��������
| 1 |
| 3 |
��ѡBC
���������ⷴӦ�����������ı仯�ǽ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��I���������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��1��Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩���������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺

���Ϸ�Ӧ�У�����ܵ��� ��ԭ����������ߵ��� ��
��3��Ϊ̽����CO2������ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺
�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��
CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H=��49.0kJ/mol
CH3OH��g��+H2O��g����H=��49.0kJ/mol
���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
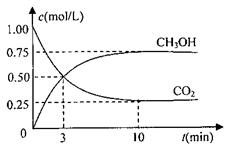
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��= ���������������� ��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ �������¶ȣ�ƽ�ⳣ������ֵ��
�����������С�����䡱����
�����д�ʩ����ʹn��CH3OH��/n��CO2��������� .
��II�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽ���£�
N2��g��+3H2��g�� 2NH3��g�� ��H=-93.4kJ/mol
2NH3��g�� ��H=-93.4kJ/mol
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2��H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
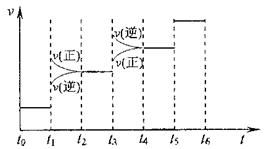

ͼt1ʱ����ƽ���ƶ������������� ��
���б�ʾƽ��������NH3������ߵ�һ��ʱ���� ��
���¶�ΪT��Cʱ����3amolH2��amolN2������ͼ��ʾ���ܱ������У�������������������ƶ�����ַ�Ӧ����N2��ת����Ϊ50%���������ͬ�¶��½�3amolH2��amolN2��2amolNH3�������������У�ƽ��ʱH2���������Ϊ ��
��1��Ŀǰ���ó��ٽ�CO2����״̬������̬��Һ̬֮�䣩���������������ѳ�Ϊһ�����ƣ���һ�����Ի����Ļ����������� ��
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺

���Ϸ�Ӧ�У�����ܵ��� ��ԭ����������ߵ��� ��
��3��Ϊ̽����CO2������ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺
�����Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��
CO2��g��+3H2��g��
 CH3OH��g��+H2O��g����H=��49.0kJ/mol
CH3OH��g��+H2O��g����H=��49.0kJ/mol���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��= ���������������� ��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ �������¶ȣ�ƽ�ⳣ������ֵ��
�����������С�����䡱����
�����д�ʩ����ʹn��CH3OH��/n��CO2��������� .
| A�������¶� | B������He��g����ʹ��ϵѹǿ���� |
| C����H2O��g������ϵ�з��� | D���ٳ���1molCO2��3molH2 |
N2��g��+3H2��g��
 2NH3��g�� ��H=-93.4kJ/mol
2NH3��g�� ��H=-93.4kJ/mol�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2��H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��


ͼt1ʱ����ƽ���ƶ������������� ��
���б�ʾƽ��������NH3������ߵ�һ��ʱ���� ��
���¶�ΪT��Cʱ����3amolH2��amolN2������ͼ��ʾ���ܱ������У�������������������ƶ�����ַ�Ӧ����N2��ת����Ϊ50%���������ͬ�¶��½�3amolH2��amolN2��2amolNH3�������������У�ƽ��ʱH2���������Ϊ ��
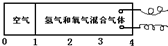 ����ͼ��ʾ���ܱ���������һ������������������磬���ܷ��Ծ��Կɿ������������������
����ͼ��ʾ���ܱ���������һ������������������磬���ܷ��Ծ��Կɿ������������������ �������У�
�������У�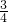 �IJ��ֳ����H2��O2�Ļ�����壻�������õ��ȼ����ʱ�������ȱ��嵽��ߣ����������ڵ������ָ���ԭ�����¶�ʱ�������������ұ��ƶ�������ͣ�������ݻ���
�IJ��ֳ����H2��O2�Ļ�����壻�������õ��ȼ����ʱ�������ȱ��嵽��ߣ����������ڵ������ָ���ԭ�����¶�ʱ�������������ұ��ƶ�������ͣ�������ݻ���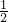 ������ԭ��������������������ȿ�����
������ԭ��������������������ȿ�����
 ��������
�������У� �IJ��ֳ���ǿ�������
�IJ��ֳ���ǿ������� �IJ��ֳ����H2��O2�Ļ�����壻�������õ��ȼ����ʱ�������ȱ��嵽��ߣ����������ڵ������ָ���ԭ�����¶�ʱ�������������ұ��ƶ�������ͣ�������ݻ���
�IJ��ֳ����H2��O2�Ļ�����壻�������õ��ȼ����ʱ�������ȱ��嵽��ߣ����������ڵ������ָ���ԭ�����¶�ʱ�������������ұ��ƶ�������ͣ�������ݻ��� ������ԭ��������������������ȿ����ǣ� ��
������ԭ��������������������ȿ����ǣ� ��