��Ŀ����
ˮ����ͨ�����ȵĽ�̿��õ��Ļ����������Щ�ɷ֣�ij��ѧ��ȤС������ʦ��ָ���£��Դ˽�����ʵ��̽����
��������롿
�ٸû������ֻ����һ����̼������
�ڸû�����庬��һ����̼��������̼��������ˮ����
�۸û������ֻ���ж�����̼��������ˮ����
�ܸû������ֻ����һ����̼��������̼������
���������ϡ�a����ˮ����ͭ��ˮ�ɰ�ɫ��Ϊ��ɫ��
b����ʯ���ǹ����������ƺ������ƵĻ���
c��Ũ�������ǿ�ҵ���ˮ�ԣ�������ijЩ����ĸ����
��ʵ����̡�ͬѧ������ʦ��ָ�����������ͼ1��ʾװ�ã���������ʵ�飨���ּг���������ȥ��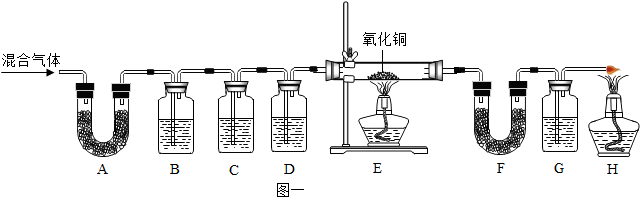
��1��װ��A����ˮ����ͭ������װ��B�г���ʯ��ˮ����ǣ��ɴ˵ó��Ľ���Ϊ����������� ����д���ƣ�
��2��װ��D�е�ҩƷΪŨ���ᣬ�������� ��
��3��E������ͭ��졢F����ˮ����ͭ������G�г���ʯ��ˮ����ǣ�˵����������л����ڵ������ǣ��û�ѧʽ��ʾ�� ��E�еı仯˵������ͭ���� �ԣ�д��E�з����û���Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ����ۡ����� ��ȷ��
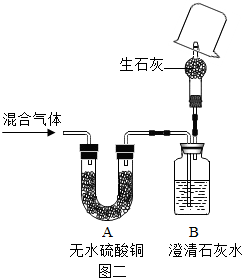
��ʵ�鷴˼����4���������ۣ�ͬѧ�ǽ�ͼ1��װ��C��H�����˼�ֻ����ͼ2��ʾװ�ò���ѡ��Ҫ�Լ��������̽�������У�������м�ʯ�ҵ�����Ϊ ���ձ����ܹ۲쵽�������� �����һ����ʵ������������� ��
��5���ڷ�Ӧǰ��E�й���������4g���ٵ�3.4g����ʣ������ͭ������Ϊ g��
��������롿
�ٸû������ֻ����һ����̼������
�ڸû�����庬��һ����̼��������̼��������ˮ����
�۸û������ֻ���ж�����̼��������ˮ����
�ܸû������ֻ����һ����̼��������̼������
���������ϡ�a����ˮ����ͭ��ˮ�ɰ�ɫ��Ϊ��ɫ��
b����ʯ���ǹ����������ƺ������ƵĻ���
c��Ũ�������ǿ�ҵ���ˮ�ԣ�������ijЩ����ĸ����
��ʵ����̡�ͬѧ������ʦ��ָ�����������ͼ1��ʾװ�ã���������ʵ�飨���ּг���������ȥ��


��1��װ��A����ˮ����ͭ������װ��B�г���ʯ��ˮ����ǣ��ɴ˵ó��Ľ���Ϊ�����������
��2��װ��D�е�ҩƷΪŨ���ᣬ��������
��3��E������ͭ��졢F����ˮ����ͭ������G�г���ʯ��ˮ����ǣ�˵����������л����ڵ������ǣ��û�ѧʽ��ʾ��
��ʵ����ۡ�����
��ʵ�鷴˼����4���������ۣ�ͬѧ�ǽ�ͼ1��װ��C��H�����˼�ֻ����ͼ2��ʾװ�ò���ѡ��Ҫ�Լ��������̽�������У�������м�ʯ�ҵ�����Ϊ
��5���ڷ�Ӧǰ��E�й���������4g���ٵ�3.4g����ʣ������ͭ������Ϊ
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,��������ļ�������ӷ���,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ̽��
��������1��������ˮ����ͭ��ˮ�����ͳ���ʯ��ˮ��������̼����ǣ��жϻ�������е����壻�����ݼ���д��ʯ��ˮ�������̼�ķ�Ӧʽ��
��2������Ũ���������ˮ�Խ��н��
��3������E������ͭ��졢F����ˮ����ͭ������G�г���ʯ��ˮ����ǣ��жϻ���������������壻
��ʵ����ۡ��������ϵ�ʵ����۶Ժż��ɣ�
��ʵ�鷴˼�����ݼ�ʯ���ǹ����������ƺ������ƵĻ���������жϣ�����ʣ���������һ����̼����ȼ���ж��ձ��е���������һ��ʵ�������
��5�����ݷ�Ӧǰ����ٵ��Dzμӷ�Ӧ������ͭ����Ԫ�ص��������н��
��2������Ũ���������ˮ�Խ��н��
��3������E������ͭ��졢F����ˮ����ͭ������G�г���ʯ��ˮ����ǣ��жϻ���������������壻
��ʵ����ۡ��������ϵ�ʵ����۶Ժż��ɣ�
��ʵ�鷴˼�����ݼ�ʯ���ǹ����������ƺ������ƵĻ���������жϣ�����ʣ���������һ����̼����ȼ���ж��ձ��е���������һ��ʵ�������
��5�����ݷ�Ӧǰ����ٵ��Dzμӷ�Ӧ������ͭ����Ԫ�ص��������н��
����⣺��1������ˮ����ͭ��ˮ�����ͳ���ʯ��ˮ��������̼����ǵ��ص��֪�������������ˮ�����Ͷ�����̼���ʴ�Ϊ��ˮ�����Ͷ�����̼��
��2��Dװ������Ũ���ᣬĿ�������ջ�������е�ˮ������
�ʴ�Ϊ�����ջ�������е�ˮ������
��3��E������ͭ��죬˵����һ����̼�������������ߵĻ�����壻F����ˮ����ͭ������˵������������Ϊ������ԭ����ͭ������ˮ��G�г���ʯ��ˮ����ǣ�˵����һ����̼���壬��Ϊһ����̼��ԭ����ͭ�����ɶ�����̼��ͬʱE�еı仯˵��������ͭ���������ԣ���������������ͭ��Ӧ����ͭ��ˮ�ǵ��ʺͻ����ﷴӦ��������ĵ��ʺͻ���������û���Ӧ����Ӧ�Ļ�ѧ����ʽΪCuO+H2
Cu+H2O��
�ʴ�Ϊ��CO��H2��������CuO+H2
Cu+H2O��
��ʵ����ۡ���������ʵ���֪�������������������һ����̼��ˮ������������̼���ʲ������ȷ��
�ʴ�Ϊ���ڣ�
��ʵ�鷴˼����ʯ���ǹ����������ƺ������ƵĻ������е��������ƹ�������ն�����̼�������ƿ�����ˮ��������Ϊ����������һ����̼�������ձ�������Сˮ�飨��ˮ������������ȼ������ˮ�����Ѹ�ٽ�С�ձ���ת���������е������ʯ��ˮ��ʯ��ˮ����ǣ���һ����̼ȼ�������˶�����̼��
�ʴ�Ϊ����ȥ��������е�ˮ�����Ͷ�����̼����ˮ�飨��ˮ������Ѹ�ٽ�С�ձ���ת���������е������ʯ��ˮ��ʯ��ˮ����ǣ�
��5����μӷ�Ӧ������ͭ������Ϊx��
x��
��100%=4g-3.4g
x=3g
ʣ�������ͭ������=4g-3g=1g
��ʣ������ͭ������Ϊ1g��
���1��
��2��Dװ������Ũ���ᣬĿ�������ջ�������е�ˮ������
�ʴ�Ϊ�����ջ�������е�ˮ������
��3��E������ͭ��죬˵����һ����̼�������������ߵĻ�����壻F����ˮ����ͭ������˵������������Ϊ������ԭ����ͭ������ˮ��G�г���ʯ��ˮ����ǣ�˵����һ����̼���壬��Ϊһ����̼��ԭ����ͭ�����ɶ�����̼��ͬʱE�еı仯˵��������ͭ���������ԣ���������������ͭ��Ӧ����ͭ��ˮ�ǵ��ʺͻ����ﷴӦ��������ĵ��ʺͻ���������û���Ӧ����Ӧ�Ļ�ѧ����ʽΪCuO+H2
| ||
�ʴ�Ϊ��CO��H2��������CuO+H2
| ||
��ʵ����ۡ���������ʵ���֪�������������������һ����̼��ˮ������������̼���ʲ������ȷ��
�ʴ�Ϊ���ڣ�
��ʵ�鷴˼����ʯ���ǹ����������ƺ������ƵĻ������е��������ƹ�������ն�����̼�������ƿ�����ˮ��������Ϊ����������һ����̼�������ձ�������Сˮ�飨��ˮ������������ȼ������ˮ�����Ѹ�ٽ�С�ձ���ת���������е������ʯ��ˮ��ʯ��ˮ����ǣ���һ����̼ȼ�������˶�����̼��
�ʴ�Ϊ����ȥ��������е�ˮ�����Ͷ�����̼����ˮ�飨��ˮ������Ѹ�ٽ�С�ձ���ת���������е������ʯ��ˮ��ʯ��ˮ����ǣ�
��5����μӷ�Ӧ������ͭ������Ϊx��
x��
| 16 |
| 64+16 |
x=3g
ʣ�������ͭ������=4g-3g=1g
��ʣ������ͭ������Ϊ1g��
���1��
������������ʵ��̽������ʽ���鳣������������һ����̼��������̼��ˮ���������ʣ�ͬѧ��Ҫ�����ۺϵĻ�ѧ֪ʶ�������ô��⣮

��ϰ��ϵ�д�
�����Ŀ
������ʵ���ʵ����������������ȷ���ǣ�������
| A�����ڿ�����ȼ�շ�������ɫ���� |
| B������ɫʯ����ҺȾ�ɵ�С�����������CO2�Ӵ���ֽ����� |
| C��þȼ��ʱ�ᷢ��ҫ�۵İ� |
| D���Ѵ��л��ǵ�ľ������ʢ�������ļ���ƿ�У�ľ����ȼ |
��һ�������Ҵ�����������һ����ȫ��յ���������ȼ����Ӧ���ɶ�����̼��ˮ������һ��δ֪��X����÷�Ӧǰ�����ʵ��������±��������жϲ���ȷ���ǣ�������
| ���� | �Ҵ� | ���� | ������̼ | ˮ | X |
| ��Ӧǰ����g | 4.6 | 8 | 0 | 0 | 0 |
| ��Ӧ������g | 0 | 0 | 4.4 | 5.4 | m |
| A������m��ֵΪ2.8 |
| B��X�����Ǹ÷�Ӧ�Ĵ��� |
| C�����������������Լ���X������ |
| D������X���ܺ���̼Ԫ�� |
�������������ڻ������ǣ�������
A�� |
B�� |
C�� |
D�� |
�п���ƻ�������ڿ����л��ɵ���ɫ��Ϊ�ػ�ɫ�����в²��ԭ����ܳ������ǣ�������
| A��ƻ���к���Cu2+ |
| B��ƻ���е�Fe2+���Fe3+ |
| C��ƻ���к���Na+ |
| D��ƻ���к���OH- |