��Ŀ����
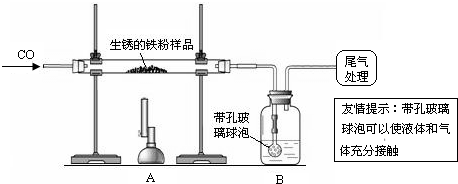
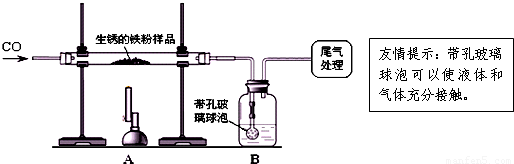
��ԭ�������ڳ�ʪ�Ŀ������������⣮����10�������������Ʒ��ij��ȤС��ͬѧ����ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ����������������

��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��
��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������B�е�����Լ���
�ٳ���ʯ��ˮ ����������Ũ��Һ ��ϡ���� ��ˮ
��3������ʵ������У�CO�������Ϊ��Ӧ���⣬�����������ǣ�
��ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը��
��ֹͣ���Ⱥ�ֹA�������ﱻ������
�۽�Aװ���е�CO2����Bװ�õ���Һ�У�
��
��4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊ8.8�ˣ�ͬʱ���װ��B����3.3�ˣ��������������Ʒ�к�����������������Ϊ

��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��
3CO+Fe2O3
2Fe+3CO2
| ||
3CO+Fe2O3
2Fe+3CO2
��
| ||
��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ��������������������B�е�����Լ���
��
��
������ţ���ʵ��ʱB�з�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH=Na2CO3+H2O
CO2+2NaOH=Na2CO3+H2O
���ٳ���ʯ��ˮ ����������Ũ��Һ ��ϡ���� ��ˮ
��3������ʵ������У�CO�������Ϊ��Ӧ���⣬�����������ǣ�
��ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը��
��ֹͣ���Ⱥ�ֹA�������ﱻ������
�۽�Aװ���е�CO2����Bװ�õ���Һ�У�
��
��������������������Ҫ�ɷ���������Ӧ��
��������������������Ҫ�ɷ���������Ӧ��
����4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊ8.8�ˣ�ͬʱ���װ��B����3.3�ˣ��������������Ʒ�к�����������������Ϊ
40%
40%
���������ǿ����ж�����̼��Ӱ�죩��������1������һ����̼���������º���������Ӧ�������Ͷ�����̼���
��2��������������������ˮ���ܺͶ�����̼��Ӧ����̼���ƺ�ˮ��������������ˮ���
��3������һ����̼��ԭ�����������ʽ��
��4������3CO+Fe2O3
2Fe+3CO2���㼴�ɽ��
��2��������������������ˮ���ܺͶ�����̼��Ӧ����̼���ƺ�ˮ��������������ˮ���
��3������һ����̼��ԭ�����������ʽ��
��4������3CO+Fe2O3
| ||
����⣺��1��һ����̼���������º���������Ӧ�������Ͷ�����̼���ʴ𰸣�3CO+Fe2O3
2Fe+3CO2��
��2����������������ˮ���ܺͶ�����̼��Ӧ����̼���ƺ�ˮ����������������ˮ��������̼��ʹ�����ʯ��ˮ����ǣ��������ն�����̼һ��ѡ���������Ƽ��������̼ѡ�ó���ʯ��ˮ������B��Ϊ�����ն�����̼���ʴ𰸣���CO2+2NaOH=Na2CO3+H2O
��3��һ����̼��ԭ�������������Ͷ�����̼���ʴ𰸣���������������������Ҫ�ɷ���������Ӧ��
��4����10g������������������Ϊx
3CO+Fe2O3
2Fe+3CO2
160 132
x 3.3g
=
x=4g
������������������������
��100%=40%
�ʴ𰸣�8����������������������Ϊ40%��
| ||
��2����������������ˮ���ܺͶ�����̼��Ӧ����̼���ƺ�ˮ����������������ˮ��������̼��ʹ�����ʯ��ˮ����ǣ��������ն�����̼һ��ѡ���������Ƽ��������̼ѡ�ó���ʯ��ˮ������B��Ϊ�����ն�����̼���ʴ𰸣���CO2+2NaOH=Na2CO3+H2O
��3��һ����̼��ԭ�������������Ͷ�����̼���ʴ𰸣���������������������Ҫ�ɷ���������Ӧ��
��4����10g������������������Ϊx
3CO+Fe2O3
| ||
160 132
x 3.3g
| 160 |
| x |
| 132 |
| 3.3g |
x=4g
������������������������
| 4g |
| 10g |
�ʴ𰸣�8����������������������Ϊ40%��
������������������������ĺ�������̽�����飬ֻҪ����������������һ����̼��Ӧ�������������̼��������Ϊװ��B��Ӧǰ�����ӵ��������ͻ��ҵ�ͻ�ƿڣ�

��ϰ��ϵ�д�
�����Ŀ
��ԭ�������ڳ�ʪ�Ŀ������������⣮����m1g�����������Ʒ��ij��ȤС��ͬѧ����ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ��������������
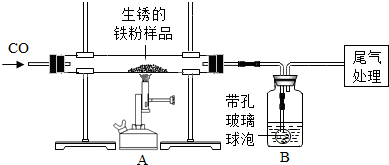

| ������ʾ�����ײ������ݿ���ʹҺ��������ֽӴ���1��װ��A�з�Ӧ�Ļ�ѧ����ʽ�� ��2��ijͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯���ⶨ����Ʒ�������������������� B�е�����Լ��� �ٳ���ʯ��ˮ ����������Ũ��Һ ��ϡ���� ��ˮ ��3������ʵ������У�CO�������Ϊ��Ӧ���⣬�����������ǣ���ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը����ֹͣ���Ⱥ�ֹA�������ﱻ������B�е���Һ������A�У��� ��4����ȫ��Ӧ��ͬѧ�Ƶ����۵�����Ϊm2g��ͬʱ���װ��B����m3g����������Ʒ������������������Ϊ
��ԭ�������ڳ�ʪ�Ŀ������������⣮����m1g�����������Ʒ��ij��ȤС��ͬѧ����ͼ��ʾװ�ý���ʵ�飬�ⶨ����Ʒ�����������������������ⰴ��������������

|