��Ŀ����
����Ŀ������������Ʒ���ִ������г����IJ��ϣ���ش��������⣺
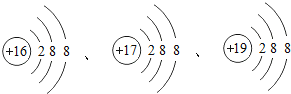
��1�������������������ˣ���Ҫ������������_____������硱���ȡ����ԡ�
��2����ĸ�����Ϳ�ϸ��ǣ���Ϊ�˷�ֹ����������_____�Ӵ�����ʴ��
��3���������Ҫ�ɷ�������������������Գ�ȥ���г���Ȧ�ϵ����⣬�䷴Ӧ�Ļ�ѧ����ʽ��_____��
��4��������ͭ���������Ļ����Һ�У�����һ�������ۣ�ʹ֮��ַ�Ӧ���ˣ������������м�������ϡ���ᣬû�������������������һ����_____���ѧʽ������Һ�е�����һ������_____���ѧʽ����
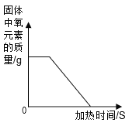
��5��ijͬѧ�ó������ⶨ����������ȼ�յIJ����![]() ����һ������������ȼ�յõ�
����һ������������ȼ�յõ�![]() ���塣��õ��Ĺ�����������_____������ĸ����
���塣��õ��Ĺ�����������_____������ĸ����
A��![]() B��
B��![]() ��
��![]() �Ļ����
�Ļ����
C��![]() ��
��![]() �Ļ���� D��
�Ļ���� D��![]() ��
��![]() ��
��![]() �Ļ����
�Ļ����
���𰸡����� ˮ������ ![]()
![]()
![]()
![]()
��������
��1�������������������ˣ���Ҫ�����������ĵ����ԣ�������ȣ�
��2����ĸ�����Ϳ�ϸ��ǣ���Ϊ�˷�ֹ����������������ˮ�Ӵ�����ʴ�����ˮ��������
��3���������Ҫ�ɷ�������������������Գ�ȥ���г���Ȧ�ϵ����⣬�䷴Ӧ�Ļ�ѧ����ʽ��Fe2O3+6HCl=2FeCl3+3H2O�����Fe2O3+6HCl=2FeCl3+3H2O��
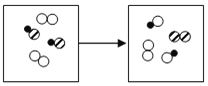
��4��������ͭ���������Ļ����Һ�У�����һ�������ۣ�ʹ֮��ַ�Ӧ���ݽ������˳����������������Ӧ����������ͭ��Ӧ�����ˣ������������м�������ϡ���ᣬû�����������˵����û�й��������Թ�����һ������������ͭ��һ��û��������Һ��һ������������������������ͭ��Ҳ�����������������Ag Fe(NO3)2��
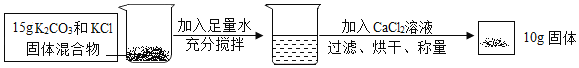
��5��11.2g����һ������������ȼ�յ�15.8g���壬�������е���Ԫ������Ϊ��15.8g-11.2g=4.6g�����������������ȣ�11.2��46=112��46�������������ﳣ������FeO��Fe2O3��Fe3O4���֣����е�������Ԫ�ص������ֱ�Ϊ��FeO�У�56��16=112��32��Fe2O3�У�112��48��Fe3O4�У�168��64��112��42.7��������������Ԫ�ص���������112��32��112��48֮�䣬���Կ�����Fe2O3��Fe3O4�����C��

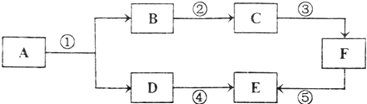
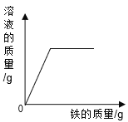
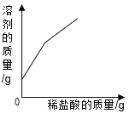
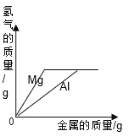
����Ŀ���±����й����ı仯ͼ�������Ӧ�����������
A | B | C | D |
|
|
|
|
��һ��������ϡ�����м����������� | ��һ������������������Һ�м���ϡ���� | ���������������������ϡ�����м���������þ���� | ���ȸ�����ع��������� |
A. AB. BC. CD. D