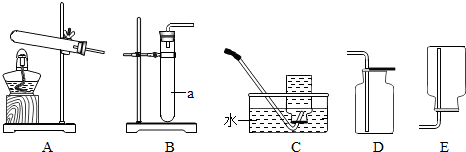
��Ŀ����
�������ͼ��ʾʵ��װ�ûش����⣺
��1��ͼ�б�Ţٵ�������
��2��Aװ�ÿ�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
��3��Bװ�ÿ�����ʵ������ȡ���ռ�������̼����Ӧ�Ļ�ѧ����ʽΪ��
��4��Cʵ������̽��ȼ�յ�������ʵ��ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա�
��������1������ͼʾд���������ƣ�
��2������ʵ�����ø��������ȡ������ԭ���Ͳ�������ش�
��3������ʵ������ȡ���ռ�������̼���������й�֪ʶ�ش������ܶȱȿ���С��������̼���ܶȱȿ�����
��4����ˮ�еİ�����Ƭ�ϵİ���ȼ������ͬ������
��2������ʵ�����ø��������ȡ������ԭ���Ͳ�������ش�
��3������ʵ������ȡ���ռ�������̼���������й�֪ʶ�ش������ܶȱȿ���С��������̼���ܶȱȿ�����
��4����ˮ�еİ�����Ƭ�ϵİ���ȼ������ͬ������
����⣺��1����������Ϊ����ƿ��
��2��������طֽ�Ļ�ѧ����ʽ2KMnO4
K2MnO4+MnO2+O2��������ˮ���ռ�������Ҫ�Ȱѵ��ܴ�ˮ���г�����Ȼ��Ϩ��ƾ��ƣ���ȷ��ʵ�����˳���� �ۢڢ٢ܢޢݣ�
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�����������ܶ�С�ڿ������ʲ����������ſ������ռ���
��4��ʵ��Ҫ�ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա� ˮ�а�����Ƭ�ϰ�������õ��ģ�
�ʴ�Ϊ��
��1������ƿ��
��2��2KMnO4
K2MnO4+MnO2+O2�����ۢڢ٢ܢޢݣ�
��3��CaCO3+2HCl�TCaCl2+H2O+CO2���� ���ܣ�
��4��ˮ�а�����Ƭ�ϰ��ף�
��2��������طֽ�Ļ�ѧ����ʽ2KMnO4
| ||
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�����������ܶ�С�ڿ������ʲ����������ſ������ռ���
��4��ʵ��Ҫ�ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա� ˮ�а�����Ƭ�ϰ�������õ��ģ�
�ʴ�Ϊ��
��1������ƿ��
��2��2KMnO4
| ||
��3��CaCO3+2HCl�TCaCl2+H2O+CO2���� ���ܣ�
��4��ˮ�а�����Ƭ�ϰ��ף�
�����������ۺ��˳��л�ѧ������Ҫ�����ʵ�����Ʒ������������й�ȼ�յ�֪ʶ��������ʵ�����Ҫ�ԣ�ͬѧ��Ҫ�ι������й��������ȡ���ռ���֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ