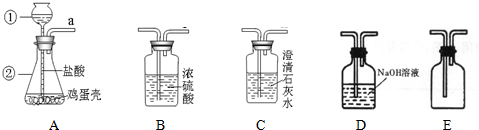
��Ŀ����
ijͬѧ����֤�������ǵ���Ҫ�ɷ���̼���Σ����ռ����������塱��ʵ�顣��������·�������ʵ�飺
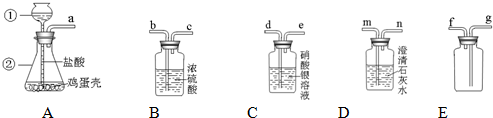
��1��д���������������ƣ��� ���� ��
��2������A��������� ��
��3������������ѧ��֪ʶ������ΪAװ�ú� װ������������ʲô����ʱ������˵�������ǵ���Ҫ�ɷ���̼���Σ� ��
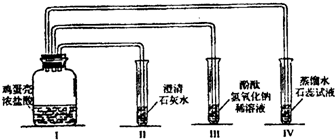
��4����ͬѧ��Ҫ�ռ�һƿ�����ĸ����壬�ٽ�һ����֤�������ʡ���������װ�õ�˳���ǣ�a��____��____��____��____��____����д���ӿ���ĸ�������У�Cװ�õ�������_
_���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��5��д����Aװ�û�������ȡ���ճ�������һ�ֳ�������Ļ�ѧ��Ӧ����ʽ�� ����˵������һ����Ҫ��; ��
1���� ����©�� ���� ��ƿ ��
��2�������Ǹ���Һ���ϣ����������ݲ������������ܽ�
��3��D Dװ���г���ʯ��ˮ�����
��4��a��__d__��__e__��__b__��__c__��g ��ȥCO2�л��е�HCl���� AgNO3 + HCl =" AgCl" ��+ HNO3
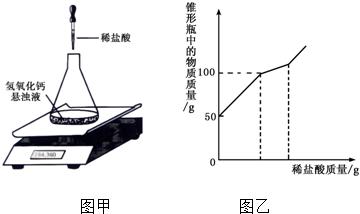
(5) 2H2O2 2H2O+O2��������������
2H2O+O2��������������
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�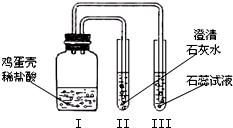 ��
��