��Ŀ����
��2007?�������Թ��ǻ�ѧʵ���еij�������������װ�����������ж�����;�����װ�ûش����⣺
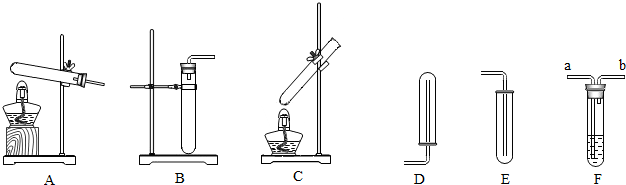
��1��ʵ�������������ȡ����������װ�ÿ�ѡ��
��2��Bװ�ÿ�����ʵ�����ƶ�����̼���䷴Ӧԭ�����û�ѧ����ʽ��ʾΪ
��3����D��Eװ���ռ�����ʱ�������ܽӽ��Թܵײ���Ŀ����
��4������һ����̼���Ƿ���ж�����̼���壬Fװ����Ӧʢ�ŵ�Һ����
��5����Ũ�����������ʱ������Ӧ��Fװ��

��1��ʵ�������������ȡ����������װ�ÿ�ѡ��
A
A
����װ�õ���ţ�����2��Bװ�ÿ�����ʵ�����ƶ�����̼���䷴Ӧԭ�����û�ѧ����ʽ��ʾΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
����3����D��Eװ���ռ�����ʱ�������ܽӽ��Թܵײ���Ŀ����
�ž�ԭ�п���
�ž�ԭ�п���
����4������һ����̼���Ƿ���ж�����̼���壬Fװ����Ӧʢ�ŵ�Һ����
����ʯ��ˮ
����ʯ��ˮ
����5����Ũ�����������ʱ������Ӧ��Fװ��
a
a
���a����b�����˵��ܽ��룮��������1��������ȡ����ķ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�
��2�����ݷ�Ӧ��������дʵ�����ƶ�����̼�ķ�Ӧԭ����
��3�����ſ������ռ�����ʱ��������Ӧ�����ӽ������ײ���Ŀ�����ž�������ʹ�ռ��������������
��4�����ó����ʯ��ˮ�����������̼���壻
��4�����������ܶȸ�������ص��������ĵ��룮
��2�����ݷ�Ӧ��������дʵ�����ƶ�����̼�ķ�Ӧԭ����
��3�����ſ������ռ�����ʱ��������Ӧ�����ӽ������ײ���Ŀ�����ž�������ʹ�ռ��������������
��4�����ó����ʯ��ˮ�����������̼���壻
��4�����������ܶȸ�������ص��������ĵ��룮
����⣺��1��ʵ�������������ȡ����������װ�ÿ�ѡ��A��
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ�Ļ�ѧ����ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2��
��3���ſ������ռ�����ʱ��������Ӧ�����ӽ������ײ���Ŀ�����ž�������ʹ�ռ��������������
��4��������̼��ʹ�����ʯ��ˮ����ǣ����ԣ�Eװ���Թ���ʢ�ŵ���Һ����������ʯ��ˮ��
��5����Ũ�����������ʱ������Ӧ�ɵ�����a�˽��룮
�ʴ�Ϊ����1��A����2��CaCO3+2HCl=CaCl2+H2O+CO2������3���ž�������ʹ�ռ����������������4����������ʯ��ˮ����5��a
��2��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼����Ӧ�Ļ�ѧ����ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2��
��3���ſ������ռ�����ʱ��������Ӧ�����ӽ������ײ���Ŀ�����ž�������ʹ�ռ��������������
��4��������̼��ʹ�����ʯ��ˮ����ǣ����ԣ�Eװ���Թ���ʢ�ŵ���Һ����������ʯ��ˮ��
��5����Ũ�����������ʱ������Ӧ�ɵ�����a�˽��룮
�ʴ�Ϊ����1��A����2��CaCO3+2HCl=CaCl2+H2O+CO2������3���ž�������ʹ�ռ����������������4����������ʯ��ˮ����5��a
����������Ƚ�ȫ��ؿ�������ȡ�����װ�õ�ѡȡ������ݷ�Ӧ���״̬�ͷ�Ӧ����ȷ������װ�ã�����������ռ�����ȷ���ռ�װ�ã�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ