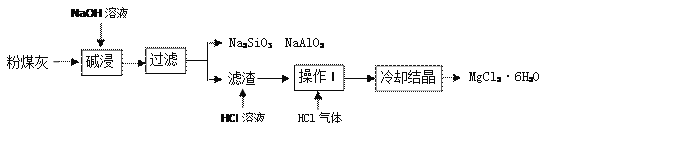
��Ŀ����
����Ŀ���������������ҹ��Ƽҵ����������°����˽��Ƽ����ư���������������Ƽ�������������ڱ��Ͱ���ˮ��(NH3��NaCl���ﵽ���͵���Һ)ͨ�� CO2��
���������ϣ�(1)�����Ƽ����Ҫ��Ӧ�� ����NaCl+NH3+CO2+H2O�TNaHCO3��+NH4Cl�� ����2NaHCO3 �� Na2CO3+H2O+CO2����
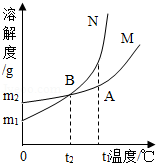
(2) NaHCO3��NH4Cl���ܽ��������ͼ��ʾ��
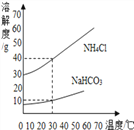
��˼��������(1)30��ʱ��NH4Cl������Һ����������������NaHCO3������Һ����������������ȣ�ǰ������ߵĹ�ϵ��_______(����ĸ)��
A������ B��С�� C������ D����ȷ��
(2)��Ӧ����������NaHCO3��NH4Cl��������Ϊ_______(���軯��)��
(3)����ˮ����CO2������NaHCO3��NH4Cl���Ƚᾧ������������NaHCO3��ԭ����________��
��ʵ��̽����ijС���Դ��κ�̼�����(NH4HCO3)Ϊԭ�ϣ��������������Ʊ������NH4Cl��
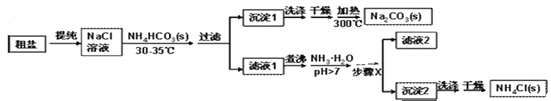
(1)�����г��˺�����ɳ�Ȳ��������ʣ�������������MgCl2�ȡ���Ҫ��ȥ�����е�MgCl2���ɼ��������NaOH��Һ�����ˣ�Ȼ������Һ�м����������ᡣд����������ʱ��Ӧ�Ļ�ѧ����ʽ_____________��
(2)����ʱ��������������______________ ��
(3)����1���泣����NH4+��Cl-�����ʣ�ϴ��ʱ���ѡ������______������Һ(����ĸ)��
A��NaCl B��NH4Cl C�� NH4HCO3 D��NaHCO3
(4)����X�����IJ�����________����ȴ�ᾧ���ˡ�
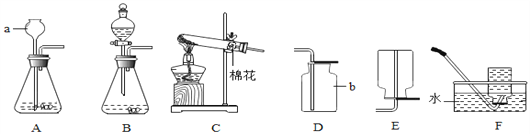
����չ���죩С�������ͼʵ��װ�òⶨij������Ʒ(����������NaCl)��̼���Ƶ�����������ȡһ��������Ʒ������ϡ���ᷴӦ��ͨ���ⶨ����CO2�������������Ʒ��̼���Ƶ�����������(��֪���³�ѹ��CO2���ܶ���1.977g/L)
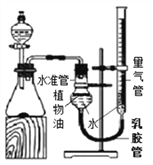
(1)�����Ʒ����Ϊ5.3g����ʵ����ѡ�õ������ܹ������ʵ���_________(����ĸ)��
A��500mL B��1000 mL C��1500 mL D��2000mL
(2) ��Ӧ��������ƿ����CO2�������ᵼ�²�õ�̼������������_________(����ĸ)��
A��ƫ�� B��ƫС C����Ӱ��
(3)���5.3g��Ʒ����Ԫ�ص���������Ϊ42%��ʵ���������ƿ����Һ���ɣ������ù�������Ϊ__________(�������1λС��)��
���𰸡� A 84:53.5 ��Ӧ���ɵ�̼�����Ƶ��������Ȼ�臨࣬����ͬ�¶���̼�����Ƶ��ܽ�ȱ��Ȼ��С NaOH+HCl=NaCl+H2O ���� D ����Ũ��(������) C C 5.7 g(��5.6 g)
���������� (2) ��˼��������(1) �ܽ����һ���¶��£�100g�ܼ���ﵽ����ʱ�����ܽ�����ʵ�������������Һ���ʵ���������=�ܽ�ȡ£��ܽ��+100g����100% �� 30��ʱ���Ȼ�淋��ܽ�ȱ�̼�����Ƶ��ܽ�ȴ���NH4Cl������Һ����������������NaHCO3������Һ����������������(2)��ѧ��Ӧ�����ʵ������ȵ��ڻ�ѧ����ʽ�л�ѧ����������Է��������˻��ı��� ��ӦNaCl+NH3+CO2+H2O�TNaHCO3��+NH4Cl����������NaHCO3��NH4Cl��������Ϊ84:53.5��(3)����ˮ����CO2������NaHCO3��NH4Cl���Ƚᾧ������������NaHCO3��ԭ���Ƿ�Ӧ���ɵ�̼�����Ƶ��������Ȼ�臨࣬����ͬ�¶���̼�����Ƶ��ܽ�ȱ��Ȼ��С ����ʵ��̽����(1)�ڳ����Ȼ�þʱ�����������NaOH��Һ�����ˣ���Һ�е�����Ϊ�Ȼ��ƺ���������������Һ�м�������ʱ���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽNaOH+HCl=NaCl+H2O ��(2)����ʱ��������������������(3)����1���泣����NH4+��Cl-�����ʣ�ϴ��ʱ��Ϊ�˲�ʹ̼�������ܽ������ѡ��̼�����Ʊ�����Һ����ϴ����(4)����X�����IJ���������Ũ������ȴ�ᾧ���ˡ�����չ���졿(1)�����Ʒ����Ϊ5.3g�����ɻ�ѧ����ʽ��Na2CO3 + 2HCl == 2NaCl + H2O + CO2�����ɼ���������ɶ�����̼2.2g��Լ1100mL��ʵ����ѡ�õ������ܹ������ʵ���1500 mL�� (2) ��Ӧ��������ƿ�в���CO2���������ƿ��ԭ�п����������ͬ�����ᵼ�²�õĶ�����̼�����ȷ����Ӱ��̼��������������(3)̼���ƺ����ᷴӦ�����Ȼ�����������̼��ˮ�����5.3g��Ʒ����Ԫ�ص���������Ϊ42%�����ݷ�Ӧǰ��Ԫ�ص�����������������ʵ���������ƿ����Һ���ɣ����ù�������Ϊ5.3g��42%����23��58.5��100%����5.7 g(��5.6 g)��