��Ŀ����
ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ���� Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ4g��ʯͷֽ����Ʒ����20 gϡ�����4�μ�����Ʒ��(��Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦҲ������ˮ)����ַ�Ӧ���ˡ�����Ȳ���������������ʵ���������±���
Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ4g��ʯͷֽ����Ʒ����20 gϡ�����4�μ�����Ʒ��(��Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦҲ������ˮ)����ַ�Ӧ���ˡ�����Ȳ���������������ʵ���������±���
| ϡ��������� | ��һ�μ���5 g | �ڶ��μ���5 g | �����μ���5 g | ���Ĵμ���5 g |
| ʣ���������� | 3 g | 2 g | l g | 1 g |
(1)�á�ʯͷֽ����̼��Ƶ��������� ��
(2)�����ϡ�����������������(д���������)��
��2���⣺��ϡ���������ʵ�����Ϊx��
CaCO3+2HCl=CaCl2+ H2O + CO2��(1��)
100 73
1�� x
|
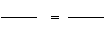 100 73
100 73 1�� x
x=0.73 g ����������������2�֣�
g ����������������2�֣�
0.73 g��5g��100%=14.6%
g��5g��100%=14.6%
��ϡ���������ʵ���������Ϊ14.6%��(1��)

��ϰ��ϵ�д�
�����Ŀ
����ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飮ʵ�����ݼ��±�������ֽ�������ɷֲ��ܽ�Ҳ����Ӧ����
��1������X��ֵΪ
��2����Ʒ��̼��Ƶ���������
��3���ձ��������ʳ�ַ�Ӧ��������Һ����Ϊ ��
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ����/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ��������/g | 10 | 20 | 30 | 40 | 50 |
| �������������/g | 0.88 | 1.76 | 2.64 | x | 3.52 |
��2����Ʒ��̼��Ƶ���������
��3���ձ��������ʳ�ַ�Ӧ��������Һ����Ϊ
����ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ��Ӧ����
��1������X��ֵΪ ��
��2������Ʒ��̼��Ƶ�����������
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
| ��ַ�Ӧ���������������/g | 0.88 | 1.76 | X | 3.52 | 3.52 |
��2������Ʒ��̼��Ƶ�����������
����ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ��Ӧ����
����Ʒ��̼��Ƶ�����������
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
| �������������/g | 0.88 | 1.76 | 2.64 | 3.52 | 3.52 |
����ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ��Ӧ����
��1��10g��Ʒ������ϡ���ᷴӦ������� g���壻
��2������Ʒ��̼��Ƶ�����������
��3���ձ��������ʳ�ַ�Ӧ��������Һ������Ϊ g��
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
| ��ַ�Ӧ���������������/g | 0.88 | 1.76 | 2.64 | 3.52 | 3.52 |
��2������Ʒ��̼��Ƶ�����������
��3���ձ��������ʳ�ַ�Ӧ��������Һ������Ϊ