��Ŀ����
��2011?������һģ������ȫ���˴����Э����ʹ����һ�ֺ�̼��Ƶġ�ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ��Ӧ����
��1������Ʒ��̼��Ƶ�����������
��2��ʵ��������ϡ�������������������
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
| �������������/g | 0.88 | 1.76 | 2.64 | 3.52 | 3.52 |
��2��ʵ��������ϡ�������������������
��������1������ʵ���¼���ݣ��ж�̼�����ȫ��Ӧʱ���ų�������̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ���ɴ�ʱ���ɶ�����̼�������������Ʒ������̼��Ƶ�������
��2�����������ж�������ȫ��Ӧ�����ɶ�����̼֮���������ϵ���ɶ�����̼����������������������������
��2�����������ж�������ȫ��Ӧ�����ɶ�����̼֮���������ϵ���ɶ�����̼����������������������������
����⣺����һ��1���ɱ������ݷ�����֪10g��Ʒ��������ϡ���ᷴӦ����CO2������Ϊ3.52g��
����Ʒ��CaCO3����������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100������������ 44
10gx�������� 3.52g����
=
x=80%����
��2���ɱ������ݷ�����֪10g������ȫ��Ӧ����0.88g������̼
�������������������Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
������73����������44
������10gy 0.88g
=
y=14.6%
��������������������ÿ10g������̼�����ȫ��Ӧ����0.88g������̼��40g������ȫ��Ӧ����3.52g������̼���ձ�������������Ϊ50g�������ɵĶ�����̼������Ϊ3.52g��˵���ڢܸ��ձ���Ʒ�е�̼�����ȫ��Ӧ����40g������10g��Ʒ�е�̼�����ȫ��Ӧ��
��̼��Ƶ���������Ϊx�������������������Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 73 44
10gx 40gy 3.52g
=
=
x=80% y=14.6%
�𣺣�1����ʯͷֽ����Ʒ��CaCO3����������Ϊ80%��
��2��ϡ�����������������Ϊ14.6%
�ʴ�Ϊ����1��80% ��2��14.6%
����Ʒ��CaCO3����������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100������������ 44
10gx�������� 3.52g����
| 100 |
| 10gx |
| 44 |
| 3.52g |
x=80%����
��2���ɱ������ݷ�����֪10g������ȫ��Ӧ����0.88g������̼
�������������������Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
������73����������44
������10gy 0.88g
| 73 |
| 10gy |
| 44 |
| 0.88g |
y=14.6%
��������������������ÿ10g������̼�����ȫ��Ӧ����0.88g������̼��40g������ȫ��Ӧ����3.52g������̼���ձ�������������Ϊ50g�������ɵĶ�����̼������Ϊ3.52g��˵���ڢܸ��ձ���Ʒ�е�̼�����ȫ��Ӧ����40g������10g��Ʒ�е�̼�����ȫ��Ӧ��
��̼��Ƶ���������Ϊx�������������������Ϊy
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 73 44
10gx 40gy 3.52g
| 100 |
| 10gx |
| 73 |
| 40gy |
| 44 |
| 3.52g |
x=80% y=14.6%
�𣺣�1����ʯͷֽ����Ʒ��CaCO3����������Ϊ80%��
��2��ϡ�����������������Ϊ14.6%
�ʴ�Ϊ����1��80% ��2��14.6%
����������ʵ���¼��������ϡ���������仯���ɶ�����̼����������仯����ķ������ɶԷ�Ӧ���������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
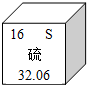 ��2011?������һģ����ͼ��Ԫ�����ڱ���ijԪ�ص���Ϣʾ��ͼ�����л�ȡ�������Ϣ����ȷ���ǣ�������
��2011?������һģ����ͼ��Ԫ�����ڱ���ijԪ�ص���Ϣʾ��ͼ�����л�ȡ�������Ϣ����ȷ���ǣ�������