��Ŀ����
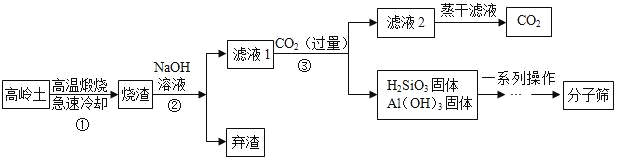
����Ŀ����ѧ��Ӧ�ڷ��λ�����Ⱦ�а�������Ҫ��ɫ��ij���������е�SO2�� �������·�ʽ��������ʽһ:![]() ����ʽ��:
����ʽ��:![]() ���Լ���:
���Լ���:
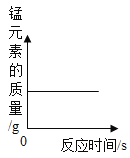
��1��CaSO4��������Ԫ�ص�������������������ϵ��������Ԫ������Ԫ�غ�_______________________��
��2�����÷�ʽһ����19. 2tSO2���ɵõ����������_______________?
��3�����÷�ʽ������19.2�� SO2, �պ���ȥ176t һ��Ũ�ȵ�NaOH��Һ����������Һ��������������______________________(���ս����ȷ��0. 1%)��
���𰸡���Ԫ�� 40.8t 21.3%
��������
��1��CaSO4��������Ԫ�ص�������������������ϵ��Ϊ32����16��4��=1��2��������Ԫ������Ԫ�غ� ��Ԫ�أ�
��2�����÷�ʽһ����19.2tSO2�����ɵ�����Ƶ�����Ϊx��
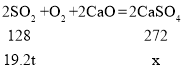
![]() ��
��
x=40.8t��
��3�����÷�ʽ������19.2tSO2�������ɵ������Ƶ�����Ϊy�����ĵ���������Ϊz��
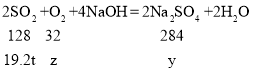
![]() y=42.6t��
y=42.6t��
![]() z=4.8t��
z=4.8t��
������Һ��������������Ϊ![]() ��100%=21.3%��
��100%=21.3%��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
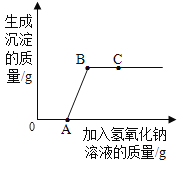
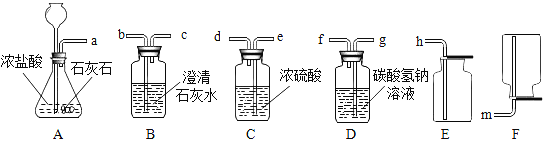
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�����Ŀ��С����ͼA��ʾװ�öԶ�����̼�����ʵ�����̽�����۲쵽��������_____��ʵ��Ľ�����_____
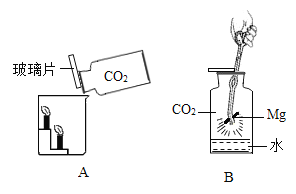
��������⣩������̼�ܷ�֧��ȼ�գ�
��������裩þ���ڶ�����̼��ȼ�ա�
���������ϣ�������þ��������þ���ǰ�ɫ������ˮ�Ĺ��塣
��MgO��2HCl=MgCl2��H2O
��MgCl2��2NaOH=Mg��OH��2����2NaCl
��ʵ��̽������ͼ��þ������ȼ�գ�ð���̣��к�ɫ�������ɣ����ų��������ȡ�
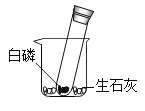
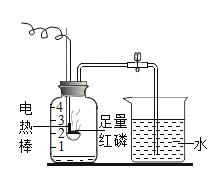
��Ϊ����������ijɷ֣�������ʵ�顣
ʵ�鲽�� | ʵ������ | ʵ����ۺͻ�ѧ����ʽ |
�����������Ĺ��ƿ�м������ϡ���ᣬ��ַ�Ӧ����ˣ�����ֽ�����к�ɫ���� | ||
��������ɫ�����ռ���ϴ�ӡ������ȼ���ڻ����Ϸ���һ��պ�г���ʯ��ˮ���ձ� | a����ɫ����ȼ�գ��ձ��ڱڳ��ְ�ɫ���� | b����ɫ������_____����Ӧ�Ļ�ѧ����ʽ�ǣ� _____��_____ |
����ȡ������Һ���Թ��У���μ�������������Һ | c����ʼ_____�����а�ɫ�������� | d�����̵ijɷ���_____ |
����˼��ߣ�ʵ������ijЩ���ý���������Ż𣬲����ö�����̼���Ӧ��ϸɳ���