��Ŀ����
����Ŀ��(1)����98%��Ũ����(�ܶ�Ϊ1.84g/mL)����500g19.6%��ϡ���ᣬ��Ҫ98%��Ũ����____mL(�����ȷ��0.1 mL)������ˮ�������____mL��������Ͳ��ȡŨ����ʱ���Ӷ�����������������ȷ����������ϡ�����������������____19.6%(������������������������)��
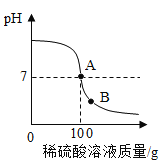
(2)��100g����������Һ�������μ�(1)�����Ƶ�ϡ���ᣬ���ò��������Ͻ��裬���pH�ı仯�����ͼ��B����Һ�е�������_____(д��ѧʽ)��
(3)A���ʾϡ���������������Һǡ����ȫ��Ӧ����A��������Һ�����ʵ���������______��(д��������̣������ȷ��0.1%)��
���𰸡�54.3 400 �� Na2SO4��H2SO4 14.2%
��������
�⣺��1������Ҫ98%��Ũ��������Ϊm��
98%m=500g��19.6%����m=100g����Ҫ98%��Ũ�������Ϊ![]() 54.3mL��
54.3mL��
��Ҫˮ������Ϊ500g-100g=400g����Ҫˮ�����Ϊ![]() =400mL��
=400mL��
������Ͳ��ȡŨ����ʱ���Ӷ�������ʵ����ȡŨ��������ƫ��������������ȷ����������ϡ�������������������19.6%��
��2���������������ᷴӦ��2NaOH��H2SO4=Na2SO4��2H2O,�����μ������������B����Һ�е������У�Na2SO4��H2SO4��
��3���裺������������Һ�����ʵ�����Ϊx��

![]() x=28.4g
x=28.4g
��������Һ��������������=![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�