��Ŀ����
����Ŀ��ʵ���У�С��������������Һ������μӵ��������У��������Ҳ�����ݲ�����Ϊ�˽������ijɷ֣�ʵ��С�����������̽�����
��������룩С������������� С�������������� С������Ƕ�����̼��
����ͬѧ��ΪС��IJ����Ǵ���ģ�����ʵ����֤��������_________��
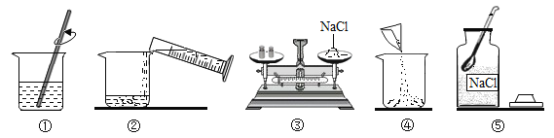
������ʵ�飩����ͼI��ʾ��װ�÷ֱ����ʵ�飺
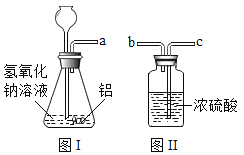
��1��С�죺��Ӧһ��ʱ���������ľ������a�����۲쵽________�������IJ������
��2��С��������ͼII��ʾװ�ø����a���ܳ��������壬���ܿ�a��______������b������c�����˹ܿ����ӡ�
�ڷ�Ӧһ��ʱ�䣬��ȼ��������壬ͨ��������ķ������ó����IJ�����ȷ��
���ó����ۣ������������ƺ�ˮ��Ӧ����������ƫ�����ƣ�NaAlO2������Ӧ�Ļ�ѧ����ʽΪ_______��
���������ۣ���ͬѧ��Ϊ��С����ʵ�鲽����д��ڰ�ȫ������������_______
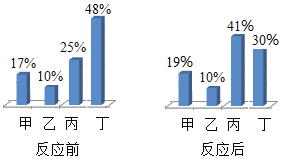
����չ��������. ij�����ķ�Һ�к���������������ͭ��Ϊ������Դ�ͷ�ֹ��Ⱦ�����ձ��м���һ������п�ۣ���Ӧֹͣ����ˣ�������ϴ�Ӹ�����ֹ���������û�иı䡣����˵����ȷ����_______(ѡ����ĸ) ��
A ������һ�������������ܺ���ͭ
B ������һ����������ͭ��һ��û��п
C ��Һ��һ����������п��һ��û��������
D ��Һ�ж���������п��һ��û������ͭ
���𰸡���ѭ�����غ㶨�ɣ�����Ӧǰ��Ԫ������� ľ�����ܸ�ȼ c 2Al+2NaOH+2H2O=2NaAlO2+3H2�� �����Ϳ�����ϴﵽһ���̶ȣ�������ը���ޣ�ʱ����ȼ�ᷢ����ը C
��������
����ͬѧ��ΪС��IJ����Ǵ���ģ�����ʵ����֤�������ǣ�ѭ�����غ㶨�ɣ�����Ӧǰ��Ԫ������䣬��Ӧ����û��̼Ԫ�أ��������ɶ�����̼���壻
��1��С�죺��Ӧһ��ʱ���������ľ������a�����۲쵽ľ�����ܸ�ȼ���϶����������������IJ������
��2��С��������ͼII��ʾװ�ø����a���ܳ��������壬���ܿ�a��c�˹ܿ����ӣ���Ũ����ϴ���������壻
�ó����ۣ������������ƺ�ˮ��Ӧ����������ƫ�����ƣ�NaAlO2������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2��
�������ۣ���ͬѧ��Ϊ��С����ʵ�鲽����д��ڰ�ȫ�����������ǣ������Ϳ�����ϴﵽһ���̶ȣ�������ը���ޣ�ʱ����ȼ�ᷢ����ը��
��չ������. ij�����ķ�Һ�к���������������ͭ��Ϊ������Դ�ͷ�ֹ��Ⱦ�����ձ��м���һ������п�ۣ���Ӧֹͣ����ˣ�������ϴ�Ӹ�����ֹ���������û�иı䣬��п���������û���ȫ����ͭ����һ�������û���Ӧ������˵����ȷ����
A��������һ����������ͭ����ѡ�����
B��������һ����������ͭ��������п����ѡ�����
C����Һ��һ����������п��һ��û������������ѡ����ȷ��
D����Һ�ж���������п������û������ͭ����ѡ�����
��ѡ��C��

����Ŀ��ij��ȤС���KClO3�ֽⷴӦ�Ĵ��������о�������ͬ�ļ��������£�����ͼװ����ɱ���ʵ�飺
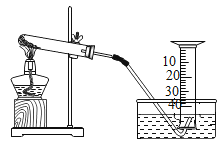
��� | KClO3����/g | ���� | ��������/g | �ռ�50mLO2����ʱ��/s |
ʵ��1 | 5 | - | - | 171 |
ʵ��2 | 5 | MnO2 | 0.5 | 49 |
ʵ��3 | 5 | Fe2O3 | 0.5 | 58 |
ʵ��4 | 5 | KCl | 0.5 | 154 |
��1������ʵ��1��Ŀ����________��
��2����������3�ִ����Ĵ�Ч����ѵ���________��
��3��д��KC1O3�ֽ�Ļ�ѧ����ʽ��________��
��4����ʵ��1��ʵ��4��֪��KCl�д����á�ά�ּ����������䣬��ʵ��1�ټ����ռ��ռ�50mLO2������ʱ����������171s������ԭ��___________��
��5��Ҫ�Ƚ�KClO3�ֽⷴӦ�в�ͬ�����Ĵ�Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ����_____________��