��Ŀ����
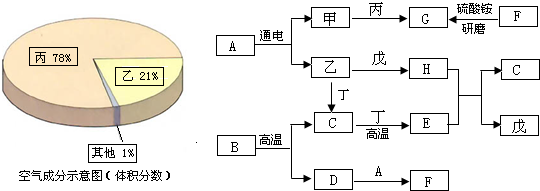
���ͼ�е����ʾ�Ϊ���л�ѧ���������ʣ�����A�ǽ������ϵ���Ҫ�ɷ֣�B�����������ͼ������֮����ת����ϵ����ش�
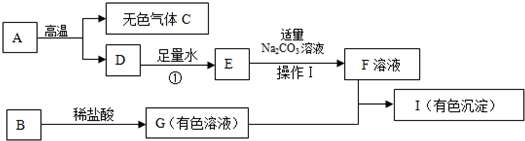
��1��д���������ʵĻ�ѧʽ��A��
��2���õ�F��Һ�IJ����������Ϊ��
��3����I�Ǻ��ɫ��������д��G+F��I�Ļ�ѧ����ʽ��
��4��ָ����Ӧ�ٵĻ���������
��5������һ���Ԫ����C��ͬ�������C��Ϻ�������������Ϊ64%����˻������10gͨ���������ʯ��ˮ��������������Ϊ

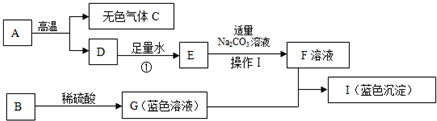
��1��д���������ʵĻ�ѧʽ��A��
CaCO3
CaCO3
C��CO2
CO2
����2���õ�F��Һ�IJ����������Ϊ��
����
����
������IJ�������©�������������ձ�
©�������������ձ�
����3����I�Ǻ��ɫ��������д��G+F��I�Ļ�ѧ����ʽ��
FeCl3+3NaOH=Fe��OH��3��+3NaCl
FeCl3+3NaOH=Fe��OH��3��+3NaCl
����4��ָ����Ӧ�ٵĻ���������
����
����
��Ӧ����5������һ���Ԫ����C��ͬ�������C��Ϻ�������������Ϊ64%����˻������10gͨ���������ʯ��ˮ��������������Ϊ
10
10
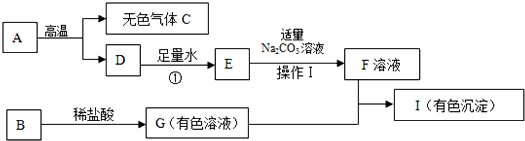
�ˣ�����������Ľ���ͻ�ƿ�����A�ǽ������ϵ���Ҫ�ɷ֣��Ҹ���ʱ�ֽܷ�������ɫ����C��D�����ݳ������ʵ����ʿ�֪������Ϊ̼��ƣ��Ӷ���˳�Ƴ�C��D��E��F���ʵĻ�ѧʽ����һͻ�ƿ�����I�Ǻ��ɫ����˵��I�������������Ӷ������Ƴ�G��B�Ļ�ѧʽ��
����C�Ƕ�����̼���������Ԫ�غ�C��ͬ�ľ���һ����̼�����Ͷ�����̼��ϣ�����Ԫ�ص���������Ϊ64%�����Լ����������̼�ĺ������Ӷ����ݻ�ѧ����ʽ��������Ӧ��̼��Ƴ�����������
����C�Ƕ�����̼���������Ԫ�غ�C��ͬ�ľ���һ����̼�����Ͷ�����̼��ϣ�����Ԫ�ص���������Ϊ64%�����Լ����������̼�ĺ������Ӷ����ݻ�ѧ����ʽ��������Ӧ��̼��Ƴ�����������
����⣺��1��A�ǽ������ϵ���Ҫ�ɷ֣��Ҹ���ʱ�ֽܷ�������ɫ����C��D�����ݳ������ʵ����ʿ�֪������Ϊ̼��ƣ���CΪ������̼��DΪ�����ƣ����Ա����Ϊ��A��CaCO3��C��CO2��
��2����������ˮ�������Ϸ�Ӧ�����������ƣ�����������̼���Ʒ�Ӧ�����������ƺ�̼��Ƴ�����Ҫ��̼��Ƴ��������ȥ�����Բ��ù��˵ķ��������˲������õ��������ܶ࣬�˴�ֻ�ʲ�������������Ӧ��Ϊ©�������������ձ������Ա����Ϊ�����ˣ�©�������������ձ���
��3��I�Ǻ��ɫ����˵��I��������������G�к��������ӣ���G���������������ᷴӦ���ɣ�˵��G���Ȼ���������G+F��I�ķ�ӦΪ�Ȼ������������Ƶķ�Ӧ���ʴ�Ϊ��FeCl3+3NaOH=Fe��OH��3��+3NaCl��
��4����Ӧ������������ˮ��Ӧ�����������ƣ�����ʽΪ��CaO+H2O�TCa��OH��2���Ƕ��һ��ʽ�ģ�Ϊ���Ϸ�Ӧ���ʴ�Ϊ�����ϣ�
��5������C�Ƕ�����̼��������C���Ԫ����ͬ��Ӧ����һ����̼����������̼��һ����̼�Ļ�����к���64%����Ԫ�أ���10g��������ж�����̼������Ϊx
������Ԫ�ص���������Ϊ64%�ɵã�
��100%����10g-x��+
��100%��x=10g��64%
x=4.4g
����4.4g������̼�������ij���ʯ��ˮ��Ӧ���ɵij���������Ϊy
CO2+Ca��OH��2�TCaCO3��+H2O
44 100
4.4g y
=
y=10g��
�ʵõ�10g������
�ʴ�Ϊ��10��
��2����������ˮ�������Ϸ�Ӧ�����������ƣ�����������̼���Ʒ�Ӧ�����������ƺ�̼��Ƴ�����Ҫ��̼��Ƴ��������ȥ�����Բ��ù��˵ķ��������˲������õ��������ܶ࣬�˴�ֻ�ʲ�������������Ӧ��Ϊ©�������������ձ������Ա����Ϊ�����ˣ�©�������������ձ���
��3��I�Ǻ��ɫ����˵��I��������������G�к��������ӣ���G���������������ᷴӦ���ɣ�˵��G���Ȼ���������G+F��I�ķ�ӦΪ�Ȼ������������Ƶķ�Ӧ���ʴ�Ϊ��FeCl3+3NaOH=Fe��OH��3��+3NaCl��
��4����Ӧ������������ˮ��Ӧ�����������ƣ�����ʽΪ��CaO+H2O�TCa��OH��2���Ƕ��һ��ʽ�ģ�Ϊ���Ϸ�Ӧ���ʴ�Ϊ�����ϣ�
��5������C�Ƕ�����̼��������C���Ԫ����ͬ��Ӧ����һ����̼����������̼��һ����̼�Ļ�����к���64%����Ԫ�أ���10g��������ж�����̼������Ϊx
������Ԫ�ص���������Ϊ64%�ɵã�
| 16 |
| 12+16 |
| 16��2 |
| 16��2+12 |
x=4.4g
����4.4g������̼�������ij���ʯ��ˮ��Ӧ���ɵij���������Ϊy
CO2+Ca��OH��2�TCaCO3��+H2O
44 100
4.4g y
| 44 |
| 4.4g |
| 100 |
| y |
y=10g��
�ʵõ�10g������
�ʴ�Ϊ��10��
�����������ͼʽ�����ƶ��⣬����Ҫ�������е���������������������ץ�ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƶϣ���һ�Ƶ����������ۣ��Ӷ��ó����ʵ���ɣ�

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ