��Ŀ����
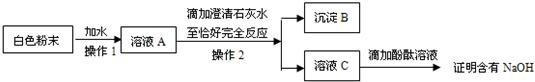
23��ijͬѧ����ʵ��ʱ����ʢ��NaOH��Һ���Լ�ƿ������Ƥ���ϳ��а�ɫ��ĩ���֣�Ϊ̽����һ��ɫ��ĩ�ijɷ֣�����������ʵ�飺
��1��ȡ������ɫ��ĩ���Թ��У��ٵμ�ϡHCl�������ݲ������ɴ˿��Ƴ���ɫ��ĩ�к���̼���ƣ�̼�����γɵĻ�ѧ����ʽ��
��2����ͬѧΪ��һ��̽����ɫ��ĩ�Ƿ���NaOH�����������ʵ�鷽����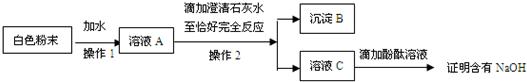
�ٳ���B����������Ϊ
�ڡ�����1����������
��������̨���ձ��Ͳ����������С�����2��ʱ�����貹��IJ���������
����ͬѧ��Ϊ�÷����еμӳ���ʯ��ˮ����ȷ����ĩ�Ƿ���NaOH����������
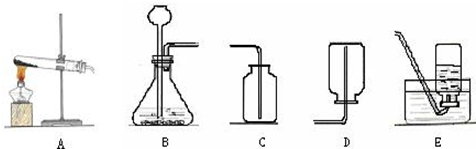
��3��Ϊ�ⶨ����������̼���Ƶ������������ɲ�������ͼ1��ʾװ�ý���ʵ�飮���мгֵ�������������Ħ��ע������ͨ����Ӧ��ע���������ռ���CO2�������������Na2CO3������������

��ͬѧ�����ͼ2����ͼ1�е��ռ�װ�ã�������CO2��������
��1��ȡ������ɫ��ĩ���Թ��У��ٵμ�ϡHCl�������ݲ������ɴ˿��Ƴ���ɫ��ĩ�к���̼���ƣ�̼�����γɵĻ�ѧ����ʽ��
2NaOH+CO2�TNa2CO3+H2O
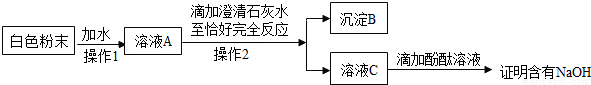
����2����ͬѧΪ��һ��̽����ɫ��ĩ�Ƿ���NaOH�����������ʵ�鷽����

�ٳ���B����������Ϊ
̼���
���ڡ�����1����������
�ܽ�
�����С�����1����Ŀ����
����Һ�����������Ʒ�Ӧ�����ڹ۲����ɵij���
����������̨���ձ��Ͳ����������С�����2��ʱ�����貹��IJ���������
©��
������ͬѧ��Ϊ�÷����еμӳ���ʯ��ˮ����ȷ����ĩ�Ƿ���NaOH����������
Na2CO3��ʯ��ˮ��Ӧ���ɵ���������Ҳ�ʼ���
�� ��ĸĽ�������
�μ��Ȼ�����Һ
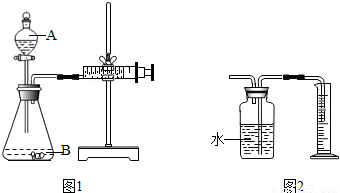
����3��Ϊ�ⶨ����������̼���Ƶ������������ɲ�������ͼ1��ʾװ�ý���ʵ�飮���мгֵ�������������Ħ��ע������ͨ����Ӧ��ע���������ռ���CO2�������������Na2CO3������������

��ͬѧ�����ͼ2����ͼ1�е��ռ�װ�ã�������CO2��������
ƫС
���ƫ����ƫС���������䡱����������
CO2������ˮ����ˮ��Ӧ
����ĸĽ�����
��ˮ���ϸ���һ��ֲ����
�����ƿ��ԭ�еĿ�����ʵ����
û��
����С���û�С���Ӱ�죮��������1���������Ʊ��������������˿����еĶ�����̼�����̼���ƣ�
��2����ͬѧ֤���������Ʋ��ֱ��ʵ�ʵ�����Ϊ��ȡ��Ʒ��ˮ�ܽ⣬��������Һ�еμ�����������Һ��̼�������������Ʒ�Ӧ����̼��Ƴ������μ����������ٲ���ʱ�����ˣ���ȥ����̼��ƣ�������С��Һ�еμӷ�̪���۲쵽��̪ ��켴�ó����庬�������Ƶ��жϣ�
��3�����ò��������������̼�ų�ˮ������ⶨ���ɶ�����̼��������������̼����������ˮ�����������̼���ܽ��ʹ��õĶ�����̼�����С��Ϊ��������Ӱ�죬��ʹ������̼��ֱ����ˮ�Ӵ���
��2����ͬѧ֤���������Ʋ��ֱ��ʵ�ʵ�����Ϊ��ȡ��Ʒ��ˮ�ܽ⣬��������Һ�еμ�����������Һ��̼�������������Ʒ�Ӧ����̼��Ƴ������μ����������ٲ���ʱ�����ˣ���ȥ����̼��ƣ�������С��Һ�еμӷ�̪���۲쵽��̪ ��켴�ó����庬�������Ƶ��жϣ�
��3�����ò��������������̼�ų�ˮ������ⶨ���ɶ�����̼��������������̼����������ˮ�����������̼���ܽ��ʹ��õĶ�����̼�����С��Ϊ��������Ӱ�죬��ʹ������̼��ֱ����ˮ�Ӵ���
����⣺��1�������������տ����еĶ�����̼��������̼���ƺ�ˮ����ʹ�������Ʊ��ʣ�
�ʴ�Ϊ��2NaOH+CO2�TNa2CO3+H2O��
��2���ٵμӵ�������������Һ��̼���Ʒ�Ӧ����̼��Ƴ�����
�ʴ�Ϊ��̼��ƣ�
�ڲ���1Ϊ�ѹ������ˮ�У���ˮ�ܽ�Ĺ��̣��˹��̳�Ϊ�ܽ⣻�����ܽ��γ���Һ�����ڹ۲�ʹ֮������������Һ��Ӧʱ���ɵ�̼��Ƴ�������������Ҫʹ�õ���Ҫ�����У�©�����ձ��Ͳ�������
�ʴ�Ϊ���ܽ⣻����Һ�����������Ʒ�Ӧ�����ڹ۲����ɵij�����©����
������������̼���Ʒ�Ӧ������̼��Ƴ������������ƣ����˺����Һ�к������ɵ��������ƣ��μӷ�̪ʱ��Һ��죬����̪���ȴ����˵��ԭ��Ĺ����к����������ƣ�
�ʴ�Ϊ��Na2CO3��ʯ��ˮ��Ӧ���ɵ���������Ҳ�ʼ��ԣ�
Ϊ������������������Ƽ����Ӱ�죬�ɰ�����������Һ�����Ȼ��Ƶ���Һ��֧̼���ƣ�
�ʴ�Ϊ���μ��Ȼ�����Һ���Ȼ��������ᱵ�ȣ�
��3��ͼ2�����������̼ͨ�뼯��ƿ��ʱ��������̼�����ܽ⣬ʹ���ų�ˮ�������С����õĶ�����̼���������ȷ��Ϊ��ֹ���ִ������ڼ���ƿ��ˮ���ϸ���һ���ͣ�����������̼��ˮ�Ӵ���
�ʴ�Ϊ��ƫС��CO2������ˮ����ˮ��Ӧ����ˮ���ϸ���һ��ֲ���ͣ����ƿ�е�ˮ��Ϊ���͵�CO2ˮ��Һ�Ⱥ����𰸣���
��װ�����ڲ����ɷ�Ӧװ���ų������壬��Ӧװ�����������˶�����̼������������ų�������ų��������Dz��Ǻ��п�������Ӱ��Զ�����̼����IJⶨ��
�ʴ�Ϊ��û�У�
�ʴ�Ϊ��2NaOH+CO2�TNa2CO3+H2O��
��2���ٵμӵ�������������Һ��̼���Ʒ�Ӧ����̼��Ƴ�����
�ʴ�Ϊ��̼��ƣ�
�ڲ���1Ϊ�ѹ������ˮ�У���ˮ�ܽ�Ĺ��̣��˹��̳�Ϊ�ܽ⣻�����ܽ��γ���Һ�����ڹ۲�ʹ֮������������Һ��Ӧʱ���ɵ�̼��Ƴ�������������Ҫʹ�õ���Ҫ�����У�©�����ձ��Ͳ�������
�ʴ�Ϊ���ܽ⣻����Һ�����������Ʒ�Ӧ�����ڹ۲����ɵij�����©����
������������̼���Ʒ�Ӧ������̼��Ƴ������������ƣ����˺����Һ�к������ɵ��������ƣ��μӷ�̪ʱ��Һ��죬����̪���ȴ����˵��ԭ��Ĺ����к����������ƣ�
�ʴ�Ϊ��Na2CO3��ʯ��ˮ��Ӧ���ɵ���������Ҳ�ʼ��ԣ�
Ϊ������������������Ƽ����Ӱ�죬�ɰ�����������Һ�����Ȼ��Ƶ���Һ��֧̼���ƣ�
�ʴ�Ϊ���μ��Ȼ�����Һ���Ȼ��������ᱵ�ȣ�
��3��ͼ2�����������̼ͨ�뼯��ƿ��ʱ��������̼�����ܽ⣬ʹ���ų�ˮ�������С����õĶ�����̼���������ȷ��Ϊ��ֹ���ִ������ڼ���ƿ��ˮ���ϸ���һ���ͣ�����������̼��ˮ�Ӵ���
�ʴ�Ϊ��ƫС��CO2������ˮ����ˮ��Ӧ����ˮ���ϸ���һ��ֲ���ͣ����ƿ�е�ˮ��Ϊ���͵�CO2ˮ��Һ�Ⱥ����𰸣���
��װ�����ڲ����ɷ�Ӧװ���ų������壬��Ӧװ�����������˶�����̼������������ų�������ų��������Dz��Ǻ��п�������Ӱ��Զ�����̼����IJⶨ��
�ʴ�Ϊ��û�У�
�������������������Ƿ���ȫ����Ϊ̼���ƣ���Ҫѡ���Ȼ��Ƶ����ʳ�ȥ������̼���ƺ��Ƿ����������ƽ��м��飮

��ϰ��ϵ�д�
�����Ŀ