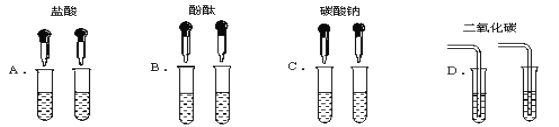
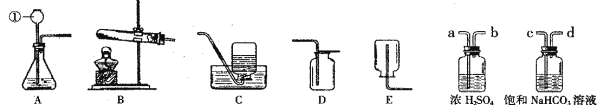
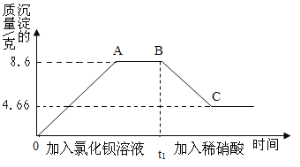
��Ŀ����
����Ŀ��ʵ����������1000g������������Ϊ4��������������Һ����ش��������⣻
��1�����Ʋ���
�ټ��㣺���������ƹ��� g��ˮ960mL(ˮ���ܶ���1 g/cm3)��
�ڳ�����������Ϊ23.1 g���ձ�������������������ƽ�ϳ�ȡ�������ƹ���ʱ��ʢ���������ƹ�����ձ�Ӧ�������̡�
���ܽ⣺���������ƹ�������ˮ���ò��������裬ʹ��������ȫ���ܽ⣬��ȴ�����¡�
��װƿ������õ���Һװ���Լ�ƿ���Ǻ�ƿ�Dz����ϱ�ǩ�������Լ����С�
����װ����������Һ���Լ�ƿ(����ͼ)��ǩ��������Ӧ����Ϣ��

��2�����ƹ��̣�������Һ������������������С��4���Ŀ���ԭ����
������Ͳ��ȡˮʱ���Ӷ�������������Һ���ձ�����������ˮ��ϴ������������ƽ��ȡ�������ƣ����������Ʒ��������̣���ʢװ��Һ���Լ�ƿ������ˮ��ϴ�����������ƹ��岻����
��3������ʦ�ṩ��ҩƷ��500 g 8��������������Һ��500 g 1��������������Һ���������������ƹ����ˮ�������������Ʒ����⣬�㻹������Ƴ���Щ���Ʒ���������д�����е�һ�֣� .(ֻҪ˵������ʱ����ĸ���ҩƷ����������)��
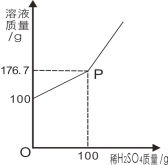
���𰸡���1���� 40 �� (������ͼ)(���ƺ�����������������ȫд����ȷ��1��) �� ��2���ڢۢܢݣ���3�� 500 g8��������������Һ���ټ�500 gˮ(��500 g 1��������������Һ���ټ�35 g�������ƹ����465 g��ˮ) (�𰸲�Ψһ����Ƶķ�����ֻҪ����40 g���������ƺ�960 gˮ���ɣ�����ֻ����������������Ϊ4��һ��Ҫ��IJ��÷�)��
��2���ڢۢܢݣ���3�� 500 g8��������������Һ���ټ�500 gˮ(��500 g 1��������������Һ���ټ�35 g�������ƹ����465 g��ˮ) (�𰸲�Ψһ����Ƶķ�����ֻҪ����40 g���������ƺ�960 gˮ���ɣ�����ֻ����������������Ϊ4��һ��Ҫ��IJ��÷�)��
��������
�����������1�����������ƣ�1000g��4%=40g����ˮ��1000g-40g=960g��960mL����������ʱӦ�������룬�Լ�ƿ��Ӧע���Լ����ƺ�������������2��������Ͳ��ȡˮʱ���Ӷ�������ȡ��ˮƫС����������������ƫ��������Һ���ձ�����������ˮ��ϴ���ᵼ��ˮ���ˣ�������������ƫС��
����������ƽ�����̳�ȡ��������ʱ�����벻����λ�þ͵�����ƽƽ�⣬�������ƶ��õ�����������ᵼ�³�����������������ƫС����ʢװ��Һ���Լ�ƿ������ˮ��ϴ���ᵼ��ˮ���ˣ�������������ƫС��
���������ƹ��岻�����ᵼ������ƫС��������������Ҳ�����ˣ���3��500g8%������������Һ�к����ʣ�500g��8%=40g����ˮ��1000g-500g=500g�����Կ�����500g8%������������Һ��500gˮ�����ơ�