��Ŀ����
 ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
[�������]��ʾ����ƿ��ɫ��Һֻ��������������Һ�е�һ�֣�
������þ��Һ������������Һ�����������Һ����������Һ
[��������]A�������£�������ʵ��ܽ�����£�
| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
��ش𣺣�1������ɫ��Һһ�����е�������______��
[ʵ��̽��]��ҩƷ����ѡ��
��2��ͨ���������ϣ�������ʵ��ܽ�ȱ�����С��ͬѧ��Ϊ����______������ţ���������ԭ����______��
��3��Ϊȷ���������ֲ����Ƿ���ȷ��С��ͬѧ���ʵ���������̽����
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ���______��Һ | ��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ�������ɫ������ | ��ҺpHС��7 | ����ܳ��� |
����д��ʵ������ٷ�Ӧ�Ļ�ѧ����ʽ______��
��������棨4��������4�֣���ȫ���ֲܷ�����60�֣�
��4���������ʵ�鷽����ȷ�ϸ���Һ���������Һ�����ʵ�鱨�棺
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ȡ����Һ�������Թ��У� ______ | ______ | ����۳������÷�Ӧ�Ļ�ѧ����ʽΪ ______ |
��2�����ݳ����������Ƶ��ܽ��Ϊ19.5g�����������ı�����Һ�����ʵ���������=
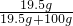 ��100%��20.0%����ˣ�����Һ�������������ƣ��ʲ���ڲ�������
��100%��20.0%����ˣ�����Һ�������������ƣ��ʲ���ڲ���������3��������þ�����ᡢ��������������У�ֻ������þ�������Һ����������Һ�γ�������þ��������ˣ��۲쵽���ְ�ɫ�����Ϳ��ж���ҺΪ����þ��Һ�����ԣ�����ٳ�����
����������Һ�����ԣ���Һ��pHС��7�����ԣ�����۳���������NH4��2SO4��ˮ��ҺҲ�����ԣ����ԣ�С��ͬѧ��ΪС��ʵ������ڵĽ��۲���ȷ�ģ���ʵ��������У�����þ���������Ʒ�Ӧ������������þ�����������ƣ���Ӧ�ķ���ʽ�ǣ�MgSO4+2 NaOH�TNa2SO4+Mg��OH��2����
��4��Ҫȷ�ϸ���Һ���������Һ�����Ƿ���笠����ӣ�����笠������������������Ӳ����̼�����ζ�İ�����������ˮ��Һ�Լ��ԣ��ܹ�ʹʪ��ĺ�ɫʯ����ֽ�����ɫ���ʲ�����������Ϊ�����Թ��м�������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��д̼�����ζ�������������ɫʯ����ֽ��������NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O
�ʴ�Ϊ����1��SO42- ��2���ڣ������£�Na2SO4������Һ����������С��20%�� ��3����NaOH�����ɣ���NH4��2SO4��ˮ��ҺҲ�����ԣ�MgSO4+2 NaOH=Na2SO4+Mg��OH��2��
��4�����Թ��м�������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ�
�д̼�����ζ�������������ɫʯ����ֽ����
��NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O
��������1�����ݲ���ı�ǩ������Һ�е����ӣ�
��2�������ܽ���뱥����Һ�����������Ĺ�ϵ������
��3����������������������þ��Ӧ���ɳ�����������٣�����pHС��7����Һ�����Է�������ܣ�
���ݣ�NH4��2SO4��ˮ��Һ�����Է���������ʵ������ٵķ�Ӧ��д����Ӧ�ķ���ʽ��
��4������笠������������������Ӳ����İ��������ʷ�����
������������ۺ��Խ�ǿ���漰����֪ʶ�ͼ��ܽ϶࣬ȷ���ձ�����Һ���������������ܽ�ȵĹ�ϵ��笠��ļ��顢��Һ�������pH��ϵ��֪ʶ���ǽ����Ĺؼ���

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д���7�֣�ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��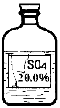
��������롿 ��ʾ����ƿ��ɫ��Һֻ��������������Һ�е�һ�֣�
������þ��Һ������������Һ�����������Һ����������Һ
���������ϡ� A�������£�������ʵ��ܽ�����£�
| ���� | MgSO4 | Na2SO4 | (NH4)2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
��ش𣺣�1������ɫ��Һһ�����е������� ��
��ʵ��̽������ҩƷ����ѡ��
��2��ͨ���������ϣ�������ʵ��ܽ�ȱ�����С��ͬѧ��Ϊ���� ������ţ���������ԭ���� ��
��3��Ϊȷ���������ֲ����Ƿ���ȷ��С��ͬѧ���ʵ���������̽����
| ʵ����� | ʵ������ | ʵ����� |
| �� ȡ����Һ�������Թ��У������еμӼ��� ��Һ | ��Һ���а�ɫ�������� | ����ٳ��� |
| �� �ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ�������ɫ������ | ��ҺpHС��7 | ����ܳ��� |
����д��ʵ������ٷ�Ӧ�Ļ�ѧ����ʽ ��
��������棨4��������4�֣���ȫ���ֲܷ�����60�֣�
��4���������ʵ�鷽����ȷ�ϸ���Һ���������Һ�����ʵ�鱨�棺
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ȡ����Һ�������Թ��У� | | ����۳������÷�Ӧ�Ļ�ѧ����ʽΪ |
��7�֣�ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
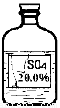
��������롿 ��ʾ����ƿ��ɫ��Һֻ��������������Һ�е�һ�֣�
������þ��Һ������������Һ�����������Һ����������Һ
���������ϡ� A�������£�������ʵ��ܽ�����£�
|
���� |
MgSO4 |
Na2SO4 |
(NH4)2SO4 |
H2SO4 |
|
�ܽ�� |
35.1g |
19.5g |
75.4g |
��ˮ����Ȼ��� |
B�� (NH4)2SO4��ˮ��Һ������
��ش𣺣�1������ɫ��Һһ�����е������� ��
��ʵ��̽������ҩƷ����ѡ��
��2��ͨ���������ϣ�������ʵ��ܽ�ȱ�����С��ͬѧ��Ϊ���� ������ţ���������ԭ���� ��
��3��Ϊȷ���������ֲ����Ƿ���ȷ��С��ͬѧ���ʵ���������̽����
|
ʵ����� |
ʵ������ |
ʵ����� |
|
�� ȡ����Һ�������Թ��У������еμӼ��� ��Һ |
��Һ���а�ɫ�������� |
����ٳ��� |
|
�� �ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ�������ɫ������ |
��ҺpHС��7 |
����ܳ��� |
С��ͬѧ��ΪС��ʵ������ڵĽ��۲���ȷ������������ ��
����д��ʵ������ٷ�Ӧ�Ļ�ѧ����ʽ ��
��������棨4��������4�֣���ȫ���ֲܷ�����60�֣�
��4���������ʵ�鷽����ȷ�ϸ���Һ���������Һ�����ʵ�鱨�棺
|
ʵ �� �� �� |
ʵ �� �� �� |
ʵ �� �� �� |
|
ȡ����Һ�������Թ��У�
|
|
����۳������÷�Ӧ�Ļ�ѧ����ʽΪ
|
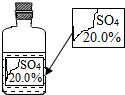
 ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��