��Ŀ����
����Ŀ�����������ʵ��װ��ͼ�ش����⡣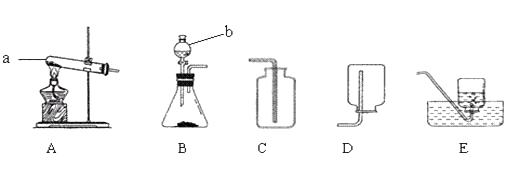
��1��д������a��b�����ƣ�a�� �� b��
��2��ʵ�����ü����Ȼ�狀��������ƹ�������ķ�����ȡ������NH3����ͬʱ�õ��Ȼ��ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ�� �� Ӧѡ�õķ���װ��Ϊ����װ�ñ�ţ���
��3���ռ�����ʱӦѡ��Dװ�ã����ռ��������ļ���ƿ�����ڵ� ����ɫ��̪��Һ��ˮ���У��۲쵽����ƿ���д�����ɫҺ����롣
����������Ϣ�ܽ�������������������ش��������ɣ���
���𰸡�
��1���Թ�,��Һ©��
��2��2NH4Cl+Ca��OH��2 ![]() CaCl2+2NH3��+2H2O,A
CaCl2+2NH3��+2H2O,A
��3���������ܶȱȿ���С,������������ˮ�γɰ�ˮ
����������1��������������ͼ��a���Թܣ�b�Ƿ�Һ©����
���Դ��ǣ��Թܣ���Һ©����
��2���Ƶð������ü����Ȼ�狀��������ƹ�������ķ��������ڡ���������͡�������ѡ��A����ѧ��Ӧ����ʽ�ǣ�2NH4Cl+Ca��OH��2 ![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
���Դ��ǣ�A��2NH4Cl+Ca��OH��2 �� _ _ CaCl2+2NH3��+2H2O��
��3���ռ�����ʱӦѡ��Dװ�ã�˵���������ܶ�С�ڿ������ܶȣ���ˮ�Լ��ԣ�������Һ��ʹ��̪��Һ��죬����ƿ���д�����ɫҺ����룬˵��������������ˮ�γɰ�ˮ��
���Դ��ǣ��������ܶȱȿ���С��������������ˮ�γɰ�ˮ��
�����㾫����������Ĺؼ�����������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ������ע�⣺a����ƽ b������ c�����ţ�

����Ŀ����pH�Ʋ��һЩҺ���pH���������������ǿ����( )
���� | θҺ | ƻ��֭ | ѪҺ | ����ˮ |
pH | 0.9��1.5 | 2.9��3.3 | 7.35��7.45 | 10��11 |
A.ƻ��֭B.θҺC.����ˮD.ѪҺ
