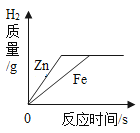
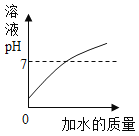
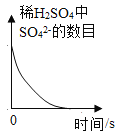
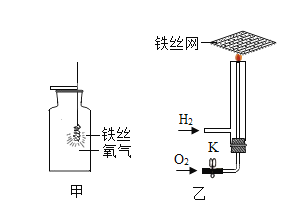
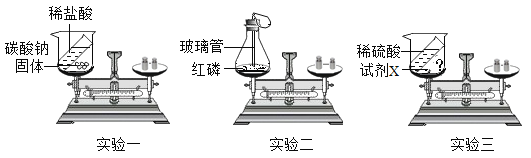
��Ŀ����
����Ŀ������ʦ��ָ���£���ѧ��ȤС����������װ�ý���������ȼ��ȼ�յ���������̽��ʵ�顣
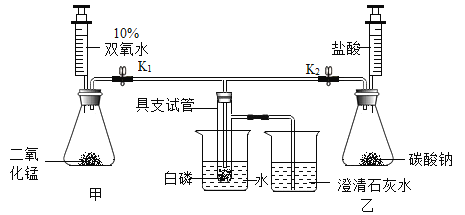
��ʵ��Ŀ�ģ�̽����ȼ��ȼ�յ�������
���������ϣ���Na2CO3+2HCl=2NaCl+H2O+CO2����
�ڰ����Ż����40�档
��ʵ�鲽�裩�ٽ�������������װ��ͼ����������
�ڴ� K1�� K2��������������һ֧ע�����Ļ�����
�۽�ʵ������ҩƷ�����Ӧ�ļס���װ���У�
�ܽ�װ���������ľ�֧�Թܷ�����ˮ�У����������������ʯ��ˮ�У�
�ݹر� K1���� K2����װ�����м������������
���ձ��е���ˮ����80�����ˮ��
��____________��
��ر�K1����K2����װ�����м������������ᡣ
�������𣩣�1��ʵ�鲽��ڵ�Ŀ����______��
��2����ʵ�鲽����У�����__________����ʵ�������жϾ�֧�Թ��������Ѿ��ž���
��3��ʵ�鲽������ܹ۲쵽����ȼ�յ������뽫����ߵIJ�����������________��
��ʵ����ۣ���1��ͨ���Ա�ʵ�鲽��_____________������ţ���ʵ�����ɵó�ȼ�յ�����֮һ�ǿ�ȼ��Ҫ�������Ӵ���
��2��ͨ���Ա�ʵ�鲽��_____________������ţ���ʵ�����ɵó�ȼ�յ���һ���������¶ȴﵽ��ȼ����Ż�㡣
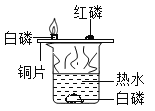
����˼�����ۣ���ʵ����̲���ȼ�յ�����̽��ʵ�飨��ͼ��ʾ����ȣ����ŵ���_____________����һ�����ɣ���
���𰸡����װ�������� ����ʯ��ˮ����� ��K1���ر�K2����װ�ü��м�������˫��ˮ �ޢ�ߢ� �ܢ� ���Է�ֹ���ɵ�������������Ⱦ�������������𰸺�����
��������
������
��1��ʵ�鲽��ڵ�Ŀ���Ǽ��װ�������ԣ�����֧�Թ��еĵ���Һ����������װ�õ����������ã�
��2����ʵ�鲽����У���������̼���뵽�ձ��У������Ѿ��ž���������̼��ʹ����ʯ��ˮ����ǣ����ݳ���ʯ��ˮ������жϾ�֧�Թ��������Ѿ��ž���
��3����ʵ���ʵ��Ŀ����̽����ȼ��ȼ�յ��������ڼ��װ�õ������Ժ���ʵ������ҩƷ���ر� K1���� K2����װ�����м������������ᣬ���ž�װ���ڵĿ������ٽ��ձ��е���ˮ����80�����ˮ��ʹ�����¶ȴﵽ���Ż�㣬��ʱ���ײ�ȼ�գ���ͨ�������Դﵽ����ȼ���������Ӷ��õ�����ȼ����Ҫ�������Ӵ�����ʵ�鲽���Ϊ����K1���ر�K2����װ�ü��м�������˫��ˮ��
ʵ�����:
��1������ޡ����а����¶ȴﵽ�Ż�㣬���������Ӵ������ײ�ȼ�գ�������а����¶ȴﵽ�Ż�㣬�������Ӵ�������ȼ�ա�ͨ���Ա�ʵ�鲽��ޢ�ߢ��ʵ�����ɵó�ȼ�յ�����֮һ�ǿ�ȼ��Ҫ�������Ӵ���
��2����������������ľ�֧�Թܷ�����ˮ�У������������Ӵ����¶�û�дﵽ�Ż�㣬���ײ�ȼ�գ�������а����¶ȴﵽ�Ż�㣬�������Ӵ�������ȼ�ա�ͨ���Ա�ʵ�鲽��ܢߵ�ʵ�����ɵó�ȼ�յ���һ���������¶ȴﵽ��ȼ����Ż�㣻
��˼�����ۣ�
��ʵ����̲���ȼ�յ�����̽��ʵ�飨��ͼ��ʾ����ȣ���ʵ�����ܱյ������ڽ��У��ŵ��ǿ��Է�ֹ���ɵ�������������Ⱦ�������������𰸺�������

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ����������Ҫ�Ľ������ϣ��ڹ�ũҵ������������Ӧ�÷dz��㷺��
I������Ӧ��
��1����������Ʒ��Ӧ��������������йص���_____��
A ���� B ���� C �˵�
��2����������-Fe������������ʳƷ���ʣ���֮Ϊ��˫����������Ϊ�������տ����е�_____��
II������ұ��
��ҵ��������Ҫ��Ӧԭ�����ڸ�����CO��ȡ����ʯ�������������ԭ��������ش��������⣺
��1��д���Գ�����Ϊԭ�ϣ��ڸ����������Ļ�ѧ����ʽ��_____��
��2����¯�����У���̿�����ó��˿�������һ����̼�⣬����_____��
�������Ļ��̽��
����һ������AgNO3��Cu ��NO3��2�����Һ������������ͼ��ʾ��ʵ�飬������ҺA����B�ijɷֽ����˷������о���
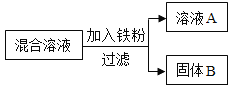
��������⣩��ҺA�е����ʳɷֿ�������Щ��
���������룩
��ֻ��Fe��NO3��2
����Fe��NO3��2��AgNO3
����Fe��NO3��2��Cu��NO3��2
����Fe��NO3��2��Cu��NO3��2��AgNO3
���������ۣ��������IJ�����_____������ţ���
��ʵ��̽���������±��е�ʵ�鲽�輰������ɱ��е�ʵ����ۡ�
ʵ�鲽�� | ���� | ʵ����� |
ȡ��������B���μ�ϡ���� | �����ݲ��� | ��ҺA�е����ʳɷַ��ϲ���_____������ţ�������B����_____�ֽ����� |
�����������������IJⶨ
ij������ȤС��ⶨ�����������ʵ��������ʼȲ�����ˮҲ�������ᣩ��������������������ȡ6����Ʒ���ֱ���ϡ���ᷴӦ������������£�������й���Ϣ�ش����⡣
ʵ����� | 1 | 2 | 3 | 4 | 5 | 6 |
ȡ��Ʒ������g�� | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
ȡϡ����������g�� | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 | 300.0 |
��������������g�� | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.05 |
��1����5��ʵ�������������Һ�����ʵĻ�ѧʽΪ_____��
��2������ϡ���������ʵ���������Ϊ_____��
��3������������Ũ�ȵ�ϡ����100�ˣ�������������Ϊ98%��Ũ����_____�ˡ�
��4���������Ʒ����������������_____����д��������̣�
����Ŀ���ܱ���������a.b.c.d�������ʣ���һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ����������
���� | a | b | c | d |
��Ӧǰ��������/g | 18 | 1 | 2 | 32 |
��Ӧ����������/g | ���� | 26 | 2 | 12 |
��1�������������ݿ�֪,�������ݵ�ֵΪ_________________���÷�Ӧ�п����Ǵ����������____________________________��
��2���÷�Ӧ��a��b֮�䷴Ӧ��������Ϊ______________��