��Ŀ����
��7 �֣�����ͼ��ʾΪʵ�����г��������Ʊ�������������ռ�������ʵ��IJ���������ijѧУ������ѧʵ��̽���С���ͬѧ����������ɸ��Ե�̽��ʵ�顣
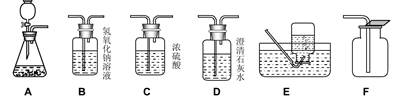
��1����һ���ͬѧ�Թ���������ҺΪԭ�ϣ�MnO2Ϊ������ ����ʵ�������Ʊ����ռ�һƿ���﴿��������������Ҫ�����ʵ��װ�� ������������������װ�õ������ԡ�
����ѡ����������˳��Ϊ �� ������д���������ĸ����
������ A ����������Ӧ�Ļ�ѧ����ʽΪ ��
����֤�����ռ����ķ����� ��
��2���ڶ����ͬѧΪ̽��������̼�����ʣ�����������ʵ�顣
�ټ�������ΪCO2Ӧѡ�����������е� ���B����D�� ���� �������Ļ�ѧ��Ӧ����ʽΪ ��
�ڽ�������̼ͨ������������Һ�У��������Է�Ӧ��������д��һ�ֻ�ѧ������֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ�������ǣ�д���Լ��������ۣ��� ��

��1����һ���ͬѧ�Թ���������ҺΪԭ�ϣ�MnO2Ϊ������ ����ʵ�������Ʊ����ռ�һƿ���﴿��������������Ҫ�����ʵ��װ�� ������������������װ�õ������ԡ�
����ѡ����������˳��Ϊ �� ������д���������ĸ����
������ A ����������Ӧ�Ļ�ѧ����ʽΪ ��
����֤�����ռ����ķ����� ��
��2���ڶ����ͬѧΪ̽��������̼�����ʣ�����������ʵ�顣
�ټ�������ΪCO2Ӧѡ�����������е� ���B����D�� ���� �������Ļ�ѧ��Ӧ����ʽΪ ��
�ڽ�������̼ͨ������������Һ�У��������Է�Ӧ��������д��һ�ֻ�ѧ������֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ�������ǣ�д���Լ��������ۣ��� ��
��1����A��C��F ��2H2O2MnO2 2H2O+ O2��
�۽������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ����˵�� O2�Ѽ�����
��2�� D �� CO2 + Ca(OH)2 = CaCO3��+ H2O
�� ȡ��Ӧ�����Һ�������Թ��У� ��������ϡ���ᣬ ������ð���� ֤�� CO2�� NaOH
��Һȷʵ�����˻�ѧ��Ӧ
�۽������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ����˵�� O2�Ѽ�����
��2�� D �� CO2 + Ca(OH)2 = CaCO3��+ H2O
�� ȡ��Ӧ�����Һ�������Թ��У� ��������ϡ���ᣬ ������ð���� ֤�� CO2�� NaOH
��Һȷʵ�����˻�ѧ��Ӧ
�����������1�����Թ���������ҺΪԭ�ϣ�MnO2Ϊ����������ʵ�������Ʊ����ռ�һƿ���﴿����������������ѡ����������˳��ΪA��C��F
������ A ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2MnO2 2H2O+ O2��
����֤�����ռ����ķ����ǣ��������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ����˵�� O2�Ѽ���
��2���ټ�������ΪCO2��Ӧ������ͨ�����ʯ��ˮ�У�Ӧѡ�����������е�D���������Ļ�ѧ��Ӧ����ʽΪ��CO2 + Ca(OH)2 = CaCO3��+ H2O
�ڣ�֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ��һ����ͨ�������Ƿ���̼�������ɣ����Է����ǣ�ȡ��Ӧ�����Һ�������Թ��У� ��������ϡ���ᣨ�������ƻ��Ȼ��ƣ��� ������ð�����а�ɫ�������ɣ��� ֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ

 A B C D E
A B C D E







 2H2O + O2������ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ���� ��
2H2O + O2������ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ���� ��