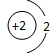
��Ŀ����
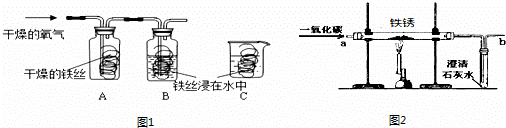
����������װ��ͼ�ش����⣺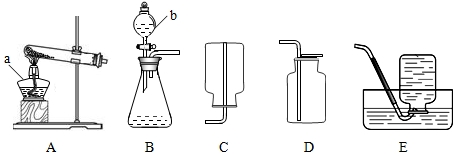
��1��д���б�����������ƣ�a
��2��ʵ������Aװ���������Ļ�ѧ����ʽ��
��ͨ�������IJ�ʵ��������ն������̣���ȷ�������Ⱥ�˳����
A�����B���ܽ�C������D��ϴ��
�ڹ��ˡ�����ʱ�����õ���������
A���ƾ���B���ձ�C��������D��©��
��3��ʵ������ȡ������̼�ķ���װ��Ϊ
���������⿼����������ȡ�е����⣮����ȡ���ռ������Ĺ����У�Ҫ�ȼ��װ�õ������ԣ����ҩƷ��ʵ������Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ��ƣ�������̼������ˮ�����Բ�������ˮ���ռ���
����⣺��1���ƾ��ƣ���Һ©����2��2KMnO4
K2MnO4+MnO2+O2����2KClO3
2KCl+3O2����3��2H2O2
2H2O+O2���������ܴ�ˮ����ȡ����Ϩ��ƾ��ƣ�BCDA��C ��3��B���ڵ������������ܺ��䵼�ܣ������������رշ�Һ©��������������һ�˽���ˮ�У���˫����ס��ƿ�������ܿ�ð�����ݣ��������Ժû��ڵ������������ܣ�����ֹˮ�У��������������Һ©���м�ˮ��������ˮ����һ����������£��������Ժã�CaCO3+2HCl�TCaCl2+CO2��+H2O�� D
| ||
| ||
| ||
������Bװ�õ������Լ���DZ�����ѵ㣬����װ�������ԵIJ�������Ϊ�ڵ������������ܺ��䵼�ܣ������������رշ�Һ©��������������һ�˽���ˮ�У���˫����ס��ƿ�������ܿ�ð�����ݣ��������Ժû��ڵ������������ܣ�����ֹˮ�У��������������Һ©���м�ˮ��������ˮ����һ����������£��������Ժã�

��ϰ��ϵ�д�
�����Ŀ
 ���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮
���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮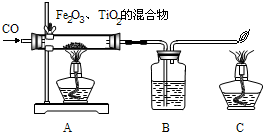
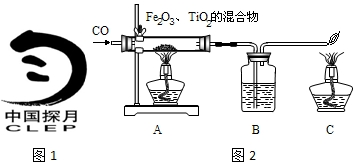
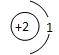 D��ԭ�ӽṹʾ��ͼΪ
D��ԭ�ӽṹʾ��ͼΪ