��Ŀ����
��ͼһ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
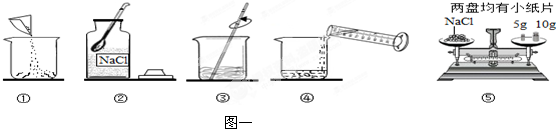
��1����ͼһ��ʾ����� ��ʾ������Һ����ȷ����˳�� ��
��2������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ�������ȡ��NaCl����Ϊ
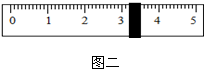
��3�����ݼ�����Ҫ��ȡˮ������� mL��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ�����߽Ƕ���ȷ���� ��ѡ����ĸ��ţ���
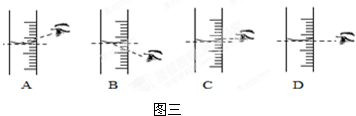
��4������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��

��1����ͼһ��ʾ����� ��ʾ������Һ����ȷ����˳��
��2������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ�������ȡ��NaCl����Ϊ

��3�����ݼ�����Ҫ��ȡˮ�������

��4������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ��������������
��������1������������Һ�IJ��裺���㡢�������ܽ�������
��2��ʹ����ƽ��ȡ�Ȼ��Ƶ�����ʱ��Ҫ��������������̣�����������̣������������=����+���룻
��3����֪���ʵ�������������������������������������Һ������������Һ������ȥ���ʾ��������ˮ���������ɽ��ʹ����Ͳ��ȡҺ��ʱ������Ӧ�밼Һ����ʹ�������ͬһˮƽ���ϣ�
��4������ȱ����һ��С�ǣ�������������С����ʹ����ȡ���Ȼ�������С��18.2g��
��2��ʹ����ƽ��ȡ�Ȼ��Ƶ�����ʱ��Ҫ��������������̣�����������̣������������=����+���룻
��3����֪���ʵ�������������������������������������Һ������������Һ������ȥ���ʾ��������ˮ���������ɽ��ʹ����Ͳ��ȡҺ��ʱ������Ӧ�밼Һ����ʹ�������ͬһˮƽ���ϣ�
��4������ȱ����һ��С�ǣ�������������С����ʹ����ȡ���Ȼ�������С��18.2g��
����⣺��1�����ƹ������ʵ���Һ�IJ����ǣ��ȼ����ٳ�������ȡ����ܽ⣬����ȷ˳���Ǣڢݢ٢ܢۣ�
��2��ʳ�ε�����=����+���룬��ͼ��֪������Ķ�����15g������Ķ�����3.2g����ʳ�ε�����=15g+3.2g=18.2g��
��3����Һ������ʳ�ε�������18.2g����Һ����Ϊ
=182g������ˮ������Ϊ182g-18.2g=163.8g��163.8mL��ʹ����Ͳ��ȡҺ��ʱ��ƽ�ӣ������밼Һ����͵㱣����ͬһˮƽ���ϣ��ʴ�Ϊ��163.8mL��©д��λ���÷֣���D��
��4��������������ȱ����һ��С�Ƕ���С���ʳƵõ��Ȼ�������С��ʵ�������������������������Һ��������С��10%��
�ʴ�Ϊ��С�ڣ�
��2��ʳ�ε�����=����+���룬��ͼ��֪������Ķ�����15g������Ķ�����3.2g����ʳ�ε�����=15g+3.2g=18.2g��
��3����Һ������ʳ�ε�������18.2g����Һ����Ϊ
| 18.2g |
| 10% |
��4��������������ȱ����һ��С�Ƕ���С���ʳƵõ��Ȼ�������С��ʵ�������������������������Һ��������С��10%��
�ʴ�Ϊ��С�ڣ�
���������⿼������Һ�����ƣ���ɴ��⣬�����������е�֪ʶ�����������������ʽ���У�

��ϰ��ϵ�д�
�����Ŀ