��Ŀ����
����Ŀ�������д����л�ѧ��������ѧ֪ʶ�ش��������⣺
(1)�ճ������г���____________�ķ�������ˮ��Ӳ�ȡ�
(2)ijѧУʳ�õ�����ṩ��������С���ࡢ���������ͷ������������Ӿ���Ӫ���ĽǶȽ���ʳ�����Ӻ�______��ʳ��(����ĸ)��
A�������� B������ C������
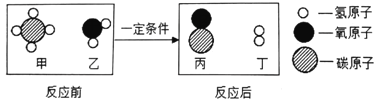
(3)�����о�֤�����ð���(NH3)�������м�ȩ(CH3OH)��ҵ��ˮ����ʹ��ת����������ʣ��йط�Ӧ�Ļ�ѧ����ʽΪ5CH3OH+12O2+6NH3![]() 3X+5CO2+19H2O����X�Ļ�ѧʽΪ______��
3X+5CO2+19H2O����X�Ļ�ѧʽΪ______��
���𰸡� ��� C N2
����������1���ճ������У�������еķ�������ˮ��Ӳ����
��2��ijѧУʳ�õ�����ṩ�������������ʡ�С���ຬ���ࡢ�������֬������ͷ�����������࣬��Ӿ���Ӫ���ĽǶȽ���ʳ�����Ӻ�ά���ص�ʳ�
��3����5CH3OH+12O2+6NH3![]() 3X+5CO2+19H2O�У���Ӧǰ����5��Cԭ�ӣ�38��Hԭ�ӣ�29��Oԭ�ӣ�6��Nԭ�ӣ���Ӧ����5��Cԭ�ӣ�38��Hԭ�ӣ�29��Oԭ�ӣ�3��X����X�к���2����ԭ�ӣ��仯ѧʽΪN2��
3X+5CO2+19H2O�У���Ӧǰ����5��Cԭ�ӣ�38��Hԭ�ӣ�29��Oԭ�ӣ�6��Nԭ�ӣ���Ӧ����5��Cԭ�ӣ�38��Hԭ�ӣ�29��Oԭ�ӣ�3��X����X�к���2����ԭ�ӣ��仯ѧʽΪN2��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ȤС��ͬѧΪ��̽��ʵ�����о��õ��������ɹ���ijɷ֣��������й�ʵ�顣
��1�����Թ�����룩
����һ��ȫ����NaOH���������ȫ����Na2CO3������������NaOH��Na2CO3�����
��2����ʵ����ƶϣ�Ϊ��һ��ȷ���ɷ֣�����������̽����
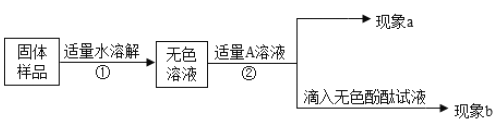
����ɫ��Һ��������ʵ�飺
ʵ����� | ʵ������ | ʵ����� |
��һ���� ���Թ�ȡԼ2mL����Һ��������������������Һ���� | ����a����ɫ���� | ��ѧ����ʽ�� ��____________________�� ��̼���Ʋ�ȫ�������� |
�ڶ����� ���ã�ȡ�ϲ���Һ���Թ��е����̪��Һ���� | ����b�� ��____________________ | ����NaOH����������������NaOH��Na2CO3����� |
��С������ͬѧ������ɣ���Ϊ����ʵ�鲻��֤�������������������ǣ�______��
�ܷ�˼���ѵ�һ���е�����������Һ����__________��Һ���ɴﵽʵ��Ŀ�ġ�
��3�������������ṩ���Լ����ṩ���Լ��У���̪��Һ��ϡ���ᡢ����������Һ���Ȼ�����Һ�������ʵ��֤������һ������������û�б��ʣ�����ȷ�ġ�����Ƶ�ʵ�鷽����__________________��