��Ŀ����
����Ŀ��������������龭�ӹ��Ƴɵİ�ɫ��ĩ������̼��ơ������ʵȳɷ֣�����Ҫ��ҩƷ����ױƷԭ�ϣ����г��ϳ��ֵļ�������������������۲�࣬����������������ǣ�
��1��Ϊ��Ѱ�������������۵ķ�������ѧ��ȤС���ͬѧ��������ͼ1ʵ�飺
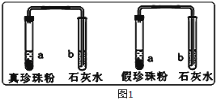
ʵ��һ���ֱ�ȡ������������������֧�Թ��У���һ������ϡ���Ტ������������ͨ�����ʯ��ˮ�У����ֳ���ʯ��ˮ������ǣ�д��ʯ��ˮ����ǵĻ�ѧ����ʽ ��˵���������۾����� ��
ʵ������ֱ�ȡ������������������Ƭ�����գ���������������ŵ��ս���ë��ζ���������δ�ŵ��ս���ë��ζ���ɴ˿ɵó����ۣ���������в��� ��
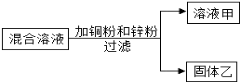
��2��Ϊ�˱Ƚ�����������̼��Ƶĺ�����������������ʵ�飬ÿ�θ�ȡ5g�������ۣ��ֱ�������ͼ2ʵ��װ����(ϡ��������������������ֻ��̼�����ϡ����ᷴӦ��������)���ⶨ������������������±���
��Ʒ �������ml | ��һ�� | �ڶ��� | ������ |
������� | 117.50 | 117.28 | 117.05 |
������� | 111.52 | 111.66 | 111.86 |
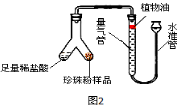
��ͼ2װ�������Եļ�鷽����________����������ˮ���Ϸ���һ��ֲ���͵�Ŀ���� ����ʵ�����ݿ�֪�����������̼��Ƶĺ��� �������(�����������������=��)��
�ڸ������β��������ȡƽ��ֵ�����5g����������ɵ���������Ϊ0.22g�������������Ʒ��CaCO3������������(д���������)
���𰸡���1��CO2+Ca(OH)2==CaCO3��+H2O CaCO3 ��������2������ͼ�еij���©�������ƶ�����ɺ��Թ��ڵ�ˮ������ˮ���ĸ߶Ȳ��䣬˵������������ ��ֹCO2����ˮ �� ��10%
��������
���������ʯ��ˮ����ǵĻ�ѧ����ʽCO2+Ca(OH)2==CaCO3��+H2O��˵���������۾�����CaCO3����������������ŵ��ս���ë��ζ���������δ�ŵ��ս���ë��ζ���ɴ˿ɵó����ۣ���������в��������ʣ�ͼ2װ�������Եļ�鷽���ǽ�ͼ�еij���©�������ƶ�����ɺ��Թ��ڵ�ˮ������ˮ���ĸ߶Ȳ��䣬˵�����������ã���������ˮ���Ϸ���һ��ֲ���͵�Ŀ���Ƿ�ֹCO2����ˮ����ʵ�����ݿ�֪�����������̼��Ƶĺ���С�ڼ�����ۣ�����Ҫ��̼��Ƶ�����ΪX��
CaCO3 + 2HCl==CaCl2 + CO2�� + H2O
100 44
X 0.22��
�б���ʽ����100��X=44��:0.22�� ���X=0.5��
�������������Ʒ��CaCO3����������=0.5�ˣ�5�ˡ�100%=10%��

 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�����Ŀ��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����

��������⡿��ƿ�Լ�������ʲô��Һ�أ�
���������ϡ�
���л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NaHCO3��
��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
�����£�20����ʱ���������ʵ��ܽ�ȵ��������£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�ܽ��g | 36 | 109 | 215 | 9��6 |
���ó����ۡ�С�������Լ�ƿ��ע��������������10%���ϱ��е��ܽ�ȵ������жϣ���ƿ�Լ��������� ��
���������롿�ٿ�����NaOH��Һ���ڿ�����Na2CO3��Һ���ۿ�����NaCl��
����Ʋ�ʵ�顿
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����PH��7������� ����
��2��СǿΪ��ȷ������Һ�ijɷݣ����ֽ���������ʵ�飺
�������� | ʵ������ | ���ۼ���ѧ����ʽ |
ȡ�����Թ��У��μ������� �����Լ������ƣ� | �������������� | �������ȷ |
��ʦָ���ý��۲����ܡ�����������������Һ�ڿ����г��ڷ��ûᷢ�����ʣ����ʺ�Ҳ�ܲ�������������д�����������ڿ����б��ʵĻ�ѧ����ʽ ��
������̽������ȡ�����������CaCl2��Һ���۲쵽�� �����������һ�����Ŀ���� �����ú�ȡ�ϲ���Һ��������ɫ��̪��Һ����Һ�ʺ�ɫ��
��ʵ����ۡ���ƿ��Һԭ���� ��
��̽����ʾ����ʵ��ʱȡ��ҩƷ��Ӧ ��
��3��̽����ƿNaOH��Һ�ı��ʳ̶�
���о�������ȡ10gԭ��Һ����������μ�����������Ϊ7.3%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȡ�
��������⡿ʵ���ü���ϡ���������CO2�����������ϵ����ͼ��ʾ��

��ش𣺢�A����Һ������������ ���ѧʽ����
��10gԭ��Һ�к�̼���Ƶ����� ����д��������̣�����2�֣�
��10gԭ��Һ��δ���ʵ��������Ƶ����� ��������Ҫд��������̣�