��Ŀ����
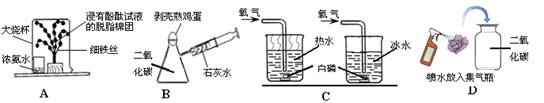
����ѧʵ��ʱ��ʵ��̨�ϵ�ҩƷӦ��������ذڷš���һ�λ�ѧ��ȤС���У�ʵ��̨�ϰڷ�������ҩƷ����1�����ᣬ��2�����ᣬ��3���������أ���4���������ƣ���5������������6�� ��7��þ����8��ͭ�����к��߿հ״����Լ�ƿ�ı�ǩ������ͼ��ʾ��

��1����1�֣����Ƚ�ͭ�����Ľ������ǿ������ѡ������ҩƷ�е�____________����ʵ�飨ѡ����ţ���
��2����2�֣�������ЩҩƷ�����ڷ��ú����ױ��ʡ��Ծ�һ����˵�����ʵ�ԭ��__________
���÷���ʽ����ʾ����
��3����7�֣�ͬѧ����̽����ǩ�����ҩƷ�ijɷ֡�
��4������˼��������ҩƷ����ڷŵ�ԭ��ҩƷ������__________��
��5���������룺A������Na2CO3��Һ B��������_________��Һ
��6����Ʋ�ʵ�飺
��С����ⶨ����Һ�����ȣ�����ʹ�����������е�_________
A ʯ����Һ B PH��ֽ C��̪��Һ
��С����ø���Һ��PHֵ����7��Сǿѡ�����ڷŵ��Լ�����С����ʵ�������ͨ����һ����ʵ��ȷ������Na2CO3��Һ�����㲹ȫСǿ��ʵ�鱨�档
��7����˼�����ۣ�Сΰ��Сǿ�Ľ���������ɣ���Ϊ���Լ���������_____________��Һ��
��Ϊ_____________��������ѧϰ����ij�������ԭ�����й�֪ʶ������Ϊ������
_____________�ķ������Լ���

��1����1�֣����Ƚ�ͭ�����Ľ������ǿ������ѡ������ҩƷ�е�____________����ʵ�飨ѡ����ţ���
��2����2�֣�������ЩҩƷ�����ڷ��ú����ױ��ʡ��Ծ�һ����˵�����ʵ�ԭ��__________
���÷���ʽ����ʾ����
��3����7�֣�ͬѧ����̽����ǩ�����ҩƷ�ijɷ֡�
��4������˼��������ҩƷ����ڷŵ�ԭ��ҩƷ������__________��
| A���� | B���� | C���� | D������ |
��6����Ʋ�ʵ�飺
��С����ⶨ����Һ�����ȣ�����ʹ�����������е�_________
A ʯ����Һ B PH��ֽ C��̪��Һ
��С����ø���Һ��PHֵ����7��Сǿѡ�����ڷŵ��Լ�����С����ʵ�������ͨ����һ����ʵ��ȷ������Na2CO3��Һ�����㲹ȫСǿ��ʵ�鱨�档
| ѡ���Լ���������ţ� | ʵ������ | ���� |
| | ������������ | ԭ�Լ���Na2CO3��Һ�� |
��Ϊ_____________��������ѧϰ����ij�������ԭ�����й�֪ʶ������Ϊ������
_____________�ķ������Լ���
��1�� ��5����8����2��Ca(OH��2 +CO2��CaCO3����H2O (3) C ̼������ AC (1)��2�� ̼������ ̼���ƺ�̼�����ƶ����Ժ���
���ϡ���ᷴӦ������������ ���Ȳ���֤�Ƿ��ж�����̼����
���ϡ���ᷴӦ������������ ���Ȳ���֤�Ƿ��ж�����̼����
��1��Ϊ�Ƚ�ͭ�����Ļ��ǿ��������ͼʾ���Լ�����ѡȡ��6���������루8��ͭ��Ӧ���м��飬��ͭ���뵽��������Һ�У�ͭ�ı���������ɫ����������˵��ͭ�Ļ����Ա���ǿ
��2��ͼʾ�е�����������Һ��������������Һ�������տ����еĶ�����̼�����ʣ��������ƺͿ����еĶ�����̼��Ӧ����̼��ƺ�ˮ���������غͿ����еĶ�����̼��Ӧ����̼��غ�ˮ
��3��[����˼��]�����ǩ�ɼ����ֱ�ʾ���������к��н�����Ԫ�أ���˿ɲ²������Ϊ����������ʣ�
[��������]��Ϊ����Ϊ�������ƣ���Ϊ�����Ϊ̼���ƻ��������Ƶȣ�
[��Ʋ�ʵ��]I���ⶨ��Һ��������PH��ֽ������������ʾ��Һ���Ի����ǿ���̶ȵģ�ʯ����Һ�ͷ�̪��Һ�ⶨ��Һ������ԣ������ܲⶨ��Һ������
II������̼���ƿ������ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壬��ˣ���ѡȡ��ͼ�Լ��е�ϡ�����ϡ���ᣬ̼������Ҳ�ܺ�ϡ�����ϡ���ᷴӦ���ɶ�����̼���壬��Сΰ��Сǿ�Ľ���������ɻ�������̼�����ƣ�̼�����������ֽ�����̼����ˮ�Ͷ�����̼
��2��ͼʾ�е�����������Һ��������������Һ�������տ����еĶ�����̼�����ʣ��������ƺͿ����еĶ�����̼��Ӧ����̼��ƺ�ˮ���������غͿ����еĶ�����̼��Ӧ����̼��غ�ˮ
��3��[����˼��]�����ǩ�ɼ����ֱ�ʾ���������к��н�����Ԫ�أ���˿ɲ²������Ϊ����������ʣ�
[��������]��Ϊ����Ϊ�������ƣ���Ϊ�����Ϊ̼���ƻ��������Ƶȣ�
[��Ʋ�ʵ��]I���ⶨ��Һ��������PH��ֽ������������ʾ��Һ���Ի����ǿ���̶ȵģ�ʯ����Һ�ͷ�̪��Һ�ⶨ��Һ������ԣ������ܲⶨ��Һ������
II������̼���ƿ������ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壬��ˣ���ѡȡ��ͼ�Լ��е�ϡ�����ϡ���ᣬ̼������Ҳ�ܺ�ϡ�����ϡ���ᷴӦ���ɶ�����̼���壬��Сΰ��Сǿ�Ľ���������ɻ�������̼�����ƣ�̼�����������ֽ�����̼����ˮ�Ͷ�����̼

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ