��Ŀ����
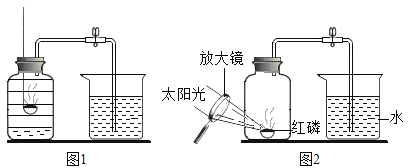
����Ŀ����ͬѧΪ̽���������ʣ������ͼ1��ʾʵ�顣
��1����ʵ���й۲쵽a�ձ�����Һ��죬�۽���Ϊ________________________��
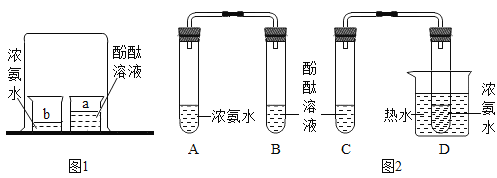
��2����ͬѧ�Լ�ͬѧ��ʵ�������ͼ2��ʾ�ĸĽ����������£�
��.��![]() ��
��![]() �Թ��зֱ����
�Թ��зֱ����![]() ������ˮ������2����ɫ��̪��Һ�����۲���Һ��ɫ
������ˮ������2����ɫ��̪��Һ�����۲���Һ��ɫ
��.��![]() ��
��![]() �Թ��зֱ����
�Թ��зֱ����![]() Ũ��ˮ�������ô���Ƥ���ĵ��ܰ�ʵ��ͼ���Ӻã�����
Ũ��ˮ�������ô���Ƥ���ĵ��ܰ�ʵ��ͼ���Ӻã�����![]() �Թܷ�����ʢ����ˮ���ձ��У��۲켸����
�Թܷ�����ʢ����ˮ���ձ��У��۲켸����
����ʵ��۲쵽��������________________���õ��Ľ�����______________��
���Աȼ�ͬѧ��ʵ�飬��ͬѧ�Ľ���ʵ����ŵ���___________________��дһ�㣩��
���𰸡������ڲ����˶� C�з�̪�ȱ�죬B�з�̪���� �¶�Խ�ߣ������˶�Խ�� ����
��������
��1���������ӻ��˶�����̪�У�ʵ���й۲쵽a�ձ�����Һ��죬�۽���Ϊ�����ڲ����˶���
��2����D�Թ��¶ȱ�A�Թܸߣ�ʵ��۲쵽��������C�з�̪�ȱ�죬B�з�̪���죬�õ��Ľ������¶�Խ�ߣ������˶�Խ�졣
�ڶԱȼ�ͬѧ��ʵ�飬��ͬѧ��ʵ�鰱�������������ͬѧ�Ľ���ʵ����ŵ��ǻ�����

����Ŀ����ͳ�ƣ��ҹ���20����90����ͷ�������Լ89���𣬸���������ش���ʧ��Ӧ�û�ѧ֪ʶ����ЧԤ���Ϳ��ƻ��֡������ͼ�����ʵ�������ԭ�����Ͳ���ȷ����( )

���ʵ�� | ���ԭ�� | |
A | סլʧ��ʱ��������Ա��ˮ��� | ���Ϳ�ȼ����Ż�� |
B | �ƾ��������Ż�ʱ����ʪĨ������ | �������������� |
C | �������Ż�ʱ���ù��Ǹ�Ϩ | �������������� |
D | ����ɭ�ֻ���ʱ�����ø���� | ��ȼ����ȼ������� |
A. A B. B C. C D. D
����Ŀ����100gBaCl2��Һ�еμ�Na2SO4��Һ����ȫ��Ӧ����Ӧ���������ɳ�����������μ�Na2SO4��Һ��������ϵ�����ʾ������Ӧ�Ļ�ѧ����ʽ��BaCl2+Na2SO4=BaSO4��+2NaCl��������㣺
�μ�Na2SO4��Һ������/g | 10 | 20 | 30 | 40 |
���ɳ���������/g | 2.33 | 4.66 | 6.99 | 6.99 |
��1��Na2SO4����Է�������Ϊ______________��
��2��ǡ����ȫ��Ӧʱ����BaSO4������Ϊ ______g
��3��BaCl2��Һ�����ʵ���������___________��д��������̣�