��Ŀ����
 ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����
ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�������1���ÿ���������ƿ�������ܵĵ������������ޡ�ɴ��������̿��С��ʯ��ʯӢɳ�Ȳ��Ͽ�����һ������ˮ��������Ϊ��������ˮ��
������
������
������ԡ������ԡ�������ˮ������Ӳˮ�����ˮ����2�������ij�����ŷŵķ�ˮ�к�������ͭ������������ͭ���ˮ��������ص���Ⱦ�������ô�ˮ���ж����ж���ԭ����
����ͭ�е�ͭ�������ؽ������ӣ��ж�
����ͭ�е�ͭ�������ؽ������ӣ��ж�
�������һ�������÷�ˮ�ķ������û�ѧ����ʽ��ʾ��CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4
CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4
����3��ʵ����Ϊ��������Һ��������ͼ1��ʾ���ұ߳��ڵ�װ�ý�����ˮ��ȡ�ɾ����̶Ƚϸߵ�����ˮ��ʹ�õ���ƿ�����ܡ��Թܵ�������ʮ�ֽྻ��ʵ�����ȷ���� ����ⷢ�ָ�����ˮ�л����к����������ʣ����е����ʿ�����
������̼
������̼
��Ϊʲô���и����ʣ��Է���ԭ��������̼������ˮ
������̼������ˮ
���ձ���ˮ������������
����
����4�����ˮʱ��Ϊ������ˮ�ĵ�������ˮ�м�ЩϡNaOH��Һ������ͨ��ֽ⺬5% NaOH ��ˮ90gһ�����ֹͣ�����ⶨ��ʱ��10% NaOH���ֽ��ˮ����Ϊ
47.4
47.4
g����������42.1
42.1
g����ʱˮ������Ӧ��
��Ӧ��
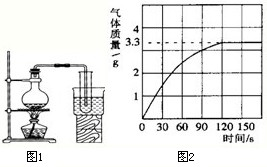
����5��ijУ��ѧ��ȤС��ͬѧΪ�˲ⶨij������Ʒ��̼���Ƶĺ�������������������Ϊ10%��������뵽10g�����У�����CO2����������ͼ2��ʾ��������Ʒ�е����ʲ������ᷴӦ����
�ٴ�ͼ�п��Կ�����10g������Ʒ�����ᷴӦ�����ɵĶ�����̼�����
3.3
3.3
g���ڴ�����̼���Ƶķ���������
79.5%
79.5%
������ʹ������ô�����Ʒǡ����ȫ��Ӧ���������������Ϊ
54.75
54.75
g��������ˮ�����ܼ�
�ܼ�
������ԡ�����ͨ����ˮϡ��ʹ10%��������5%��Ҳ����ͨ���Ӽ�������ʹ10%��������20%����������
�����������Ȼ����ӿ�ӷ���ʹ��Һ��ϡ
�����������Ȼ����ӿ�ӷ���ʹ��Һ��ϡ
���������������е�֪ʶ���з�����Ӳˮ�������Ǽ���ˮ�еĿ����Ը�þ���������ͭ���ؽ����Σ��ж�����ȥ����ͭ����ʹ������������Һ��������̼��������ˮ�����壬������������������ʽ�����йصļ��㣬��ͼ��֪���ɶ�����̼������Ϊ3.3g�����ݷ�Ӧ�Ļ�ѧ����ʽ�������̼���Ƶ����������������������������лӷ��ԣ������ܼӿ��Ȼ���Ļӷ���
����⣺��1����������ˮ�� �ܳ�ȥ���ֲ����Թ��塢��ζ��ɫ�أ����ܳ�ȥ�ܽ���ˮ�е����ʣ����ܽ�Ӳˮ��������������ԣ�
��2������ͭ���ؽ����Σ��ж�����ȥ����ͭ����ʹ������������Һ������������������ͭ��Ӧ����������ͭ����������ƣ��������ͭ�е�ͭ�������ؽ������ӣ��ж���
CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
��3����������õ�������ˮ�п��ܺ��ж�����̼����Ϊ������̼��������ˮ�����ʣ������������ʱ�ձ��е�ˮ�����������˽��µ����ã����������̼��������̼������ˮ�����£�
��4����5% NaOH ��ˮ90g������������Һ������Ϊ
���豻�ֽ��ˮ������Ϊx�����У�
��5%= (
-x)��10%�����x��47.4g�����ˮ��ˮ�����ɵ�������������Ϊ9��8������������������Ϊ47.4g��
��42.1g����ʱˮ�Ƿ�Ӧ����47.4��42.1����Ӧ�
��5���پ�ͼ��֪���ɶ�����̼������Ϊ3.3g�����3.3��
����̼���Ƶ�����Ϊx����Ҫ�����������Ϊy
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 73 44
x y 3.3g
=
x=7.95g
̼���Ƶ���������Ϊ��
��100%=79.5%
���79.5%
��
=
y=5.475g
�����������Ϊ
=54.75g��
��ʱˮ�������ܼ������54.75���ܼ���
��������лӷ��ԣ������ܼӿ��Ȼ���Ļӷ����ʼ�������ˮ�ֲ���ʹ��Һ��Ũ����������������Ȼ����ӿ�ӷ���ʹ��Һ��ϡ��
��2������ͭ���ؽ����Σ��ж�����ȥ����ͭ����ʹ������������Һ������������������ͭ��Ӧ����������ͭ����������ƣ��������ͭ�е�ͭ�������ؽ������ӣ��ж���
CuSO4+Ca��OH��2�TCu��OH��2��+CaSO4��
��3����������õ�������ˮ�п��ܺ��ж�����̼����Ϊ������̼��������ˮ�����ʣ������������ʱ�ձ��е�ˮ�����������˽��µ����ã����������̼��������̼������ˮ�����£�
��4����5% NaOH ��ˮ90g������������Һ������Ϊ
| 90g |
| 95% |
| 90g |
| 95% |
| 90g |
| 95% |
| 8 |
| 9 |
��5���پ�ͼ��֪���ɶ�����̼������Ϊ3.3g�����3.3��
����̼���Ƶ�����Ϊx����Ҫ�����������Ϊy
Na2CO3+2HCl�T2NaCl+CO2��+H2O
106 73 44
x y 3.3g
| 106 |
| x |
| 44 |
| 3.3g |
x=7.95g
̼���Ƶ���������Ϊ��
| 7.95g |
| 10g |
���79.5%
��
| 73 |
| y |
| 44 |
| 3.3g |
y=5.475g
�����������Ϊ
| 5.475g |
| 10% |
��ʱˮ�������ܼ������54.75���ܼ���
��������лӷ��ԣ������ܼӿ��Ȼ���Ļӷ����ʼ�������ˮ�ֲ���ʹ��Һ��Ũ����������������Ȼ����ӿ�ӷ���ʹ��Һ��ϡ��
���������⿼���˾�ˮ���й�֪ʶ�Լ����ݻ�ѧ����ʽ���еļ��㣬��ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ
 ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����
ˮ����Ҫ����Դ�����˼�һ����������������ģ���Ȼ���ˮ���и������ʣ�������ֱ��ʹ�ã�����о�����