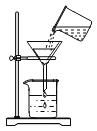
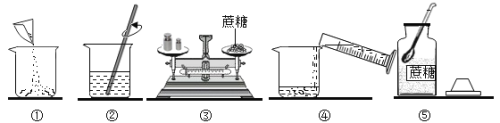
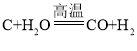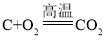��Ŀ����
����Ŀ���ֱ�����Ҫ�����:
��1��ָ�����л�ѧ���������ֵ�����:Ca2+����2��_________________________��P2O5����5�� :_____________________________��
��2���û�ѧ�������: ��3��������:________��
��2����ԭ��:______________________________��
��4�������ӣ�______________________________��
��1����ԭ��:______________________________��
����Ԫ��:______________________________��
��2������������:______________________________��
��̼�������:______________________________��
�������������Ԫ��+4��:______________________________��
��3��������мӵ�Ԫ�صĻ��ϼۣ�HNO3��AgC1��WO3�� NH3��Cu:_______
��4��д���������ʵĻ�ѧʽ:����____________________���Ȼ���____________________��������___________________��������_____________________������ͭ____________________��
���𰸡�һ�������Ӵ�2����λ����� һ������������������5����ԭ�� 3O2 2H 4Na+ Ne Al 2OH- CO32- ![]()
![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() He CaCl2 Fe2O3 ClO2 CuSO4
He CaCl2 Fe2O3 ClO2 CuSO4
��������
��1��Ca2+����2����ʾһ�������Ӵ�2����λ����ɡ�
P2O5����5����ʾһ������������������5����ԭ�ӡ�
���һ�������Ӵ�2����λ����ɣ�һ������������������5����ԭ�ӡ�
��2����3�������ӿ��Ա�ʾΪ3O2��
��2����ԭ�ӿ��Ա�ʾΪ2H��
�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ����������ӷ���ǰ������Ӧ�����֣�����4�������ӱ�ʾΪ4Na+��
��ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ���ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�����1����ԭ�ӱ�ʾΪNe��
����Ԫ�ط�������ʾԪ�أ�����Al������ʾ��Ԫ�أ�
�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ����������������ɶ��ԭ����ɵ����ӣ������1����Ԫλ�ĸ���ɣ���ʾΪOH-������ʾ��������ӣ�������Ԫ�ط���ǰ������Ӧ�����֣����2OH-��
��̼������Ӵ���2����λ�ĸ���ɣ����CO32-��
�������������Ԫ����+4�۾����ڶ�������ѧʽ����Ԫ�ط��ŵ����Ϸ�����+4�����![]() ��
��
��3��HNO3�У���Ԫ����+1�ۣ���Ԫ����-2�ۣ��赪Ԫ�صĻ��ϼ�Ϊx����+1��+x+��-2����3=0��x=+5��
AgCl�У���Ԫ����+1�ۣ�����Ԫ�صĻ��ϼ�Ϊx����+1+x=0��x=-1��
WO3�У���Ԫ����-2�ۣ���WԪ�صĻ��ϼ�Ϊx����x+��-2����3=0��x=+6��
NH3�У�HԪ����+1�ۣ���NԪ�صĻ��ϼ�Ϊx����x+��+1����3=0��x=-3��
Cu�ǽ������ʣ����ϼ�Ϊ0��
���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��
��4������ϡ������Ԫ�أ���ϡ������Ԫ����ɵĵ����ǵ�ԭ�ӷ��ӣ����Ժ����Ļ�ѧʽ���DZ�ʾ��Ԫ�ص�Ԫ�ط��ţ�����He��
�Ȼ����и�Ԫ����+2�ۣ�����-1�ۣ��仯ѧʽΪ��CaCl2��
����������Ԫ��Ϊ+3�ۣ�OԪ��Ϊ-2�ۣ����ݻ��ϼ۵Ľ����֪�������Ļ�ѧʽΪFe2O3��
���ݶ������ȵĶ�������ѧʽд����ClO2�����ClO2��
����ͭ��ͭԪ����+2�ۣ��������-2�ۣ�����ͭ�Ļ�ѧʽΪ��CuSO4��

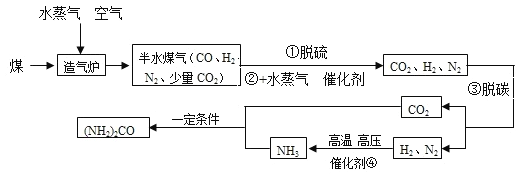
����Ŀ����̿����Ҫ�ɷ���̼���ʣ����ʲ���ˮ��Ӧ�� ��ˮ�����ڸ��������·�Ӧ���ܲ���һ���׳�Ϊˮú��������ȼ�ϣ����ܻ���H2��CO��CO2.ijС��ͬѧ���������ʵ��װ�ò�����ʵ�顣ʵ����� A װ������Һ����ǣ�D �еĹ����ɺ�ɫ���ɫ��E �еĹ����� ��ɫ����ɫ��G װ������ˮ�����ձ�������֪��ˮ����ͭ��ˮ������������ʯ�������ն�����̼��ˮ������Ũ����������ˮ��������
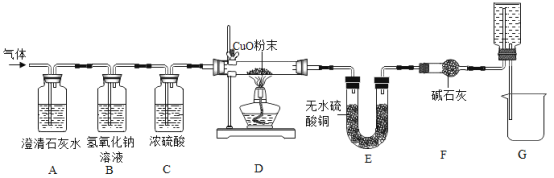
�ڷ�Ӧǰ���D��Eװ�ý����������IJ�����������Ӧ����ȫ����������������
װ�ü�ҩƷ | ��Ӧǰ | ��Ӧ�� |
Dװ���в�����������������/g | 223.3 | 215.0 |
Eװ����U��������������/g | 260.0 | 267.2 |
������ش��������⣺
��1��ˮú����һ������CO2��������_____���û�ѧ����ʽ��ʾ����
��2��һ����֤��ˮú�������������ڵ�ʵ��������_____��_____��
��3��G װ�õ����ã��١�����������_____��
��4����С��ͬѧͨ�����ݷ�����֤��ˮú���д��� CO ���壬�������ϱ��е�ԭʼ���ݣ��г����ݷ������̣�ֻ��ʽ�����㣩��_____
��5��ͬѧ�Ƿ��֣���ͨ��������Ӧǰ��װ�õ�������Ҳ�ɷ����֤�� CO ����Ĵ��ڡ� ������һ��ͼ�е�װ��_____����װ����ţ�������װ�� F��
����Ŀ��ij��ѧ��ȤС����ͨ��ʵ��ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ��ʯ��ʯ��Ʒ8.0g����80mLϡ������Ĵμ��룬�����������±�����֪��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ����
ʵ����� | 1 | 2 | 3 | 4 |
����ϡ����������mL�� | 20 | 20 | 20 | 20 |
��ַ�Ӧ��ʣ������������g�� | 5.5 | m | 1.2 | n |
��1���ϱ��У�m��_____��n��_____��
��2����ʯ��ʯ��̼�Ƶ���������Ϊ_____��
��3����Ҫ��ȡ6.6g������̼��������Ҫ��ʯ��ʯ���ٿˣ�_____��д��������̣������ȷ��0.lg��