��Ŀ����
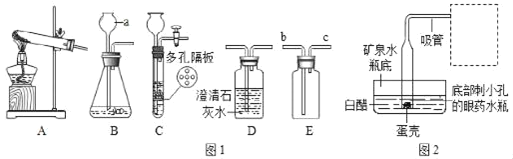
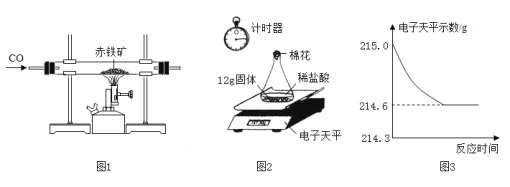
����Ŀ��ijͬѧ����ͼ1װ��ģ�ҵ��������ͨ��ʵ�飨��ͼ2���ⶨͼ1��ַ�Ӧ�����ù����е�����������������
��1��125g��������80%�ij������к�����Ԫ�ص�����Ϊ____________g��
��2��֧��ͼ1ʵ��װ�������Ե�һ��ȱ©________________��ͼ1�����Ļ�ѧ��Ӧ����ʽ__________________________________��
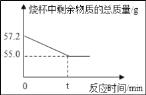
��3����ͼ1ʵ���ַ�Ӧ��ȡ��Ӧ�����12g����ƿ�У���������ϡ�����������ʼ��¼������ƽ��ʾ������¼������ͼ3�������12g�����е�����������������__________________________.
���𰸡�70 β��û�н��д���������Ⱦ���� Fe2O3+3CO ![]() 2Fe+3CO2 93.3%
2Fe+3CO2 93.3%
��������
��1��125g��������80%�ij������к�����Ԫ�ص�����Ϊ125g��80%��![]() ��100%=70g��
��100%=70g��
��2���������÷�Ӷһ����̼�ж�������ͼ1ʵ��װ�������Ե�һ��ȱ© β��û�д���������Ⱦ������ͼ1�����Ļ�ѧ��ӦΪһ����̼���������ڸ������������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe2O3+3CO ![]() 2Fe+3CO2
2Fe+3CO2
��3�����������غ㶨�ɿɵ����ɵ�����������Ϊ215g-214.6g=0.4g
12g�����е���������������Ϊx
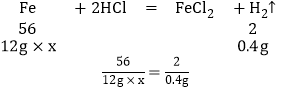
x��93.3%

 ���������ν�ϵ�д�
���������ν�ϵ�д�