��Ŀ����
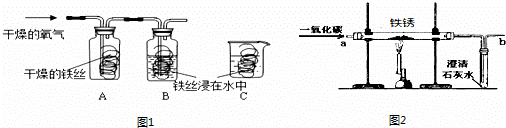
 23������������װ��ͼ�ش����⣺
23������������װ��ͼ�ش����⣺��1��ij��ѧ��ȤС���ô�װ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ�������������ǣ�
�����ܿ������ݣ�ʯ��ˮ�����
������������ķ�Ӧ�Ļ�ѧ����ʽ��CO2+Ca��OH��2�TCaCO3��+H2O
���������������ͨ�������̼����һ��ʱ����ֳ����ܽ��ɳ�����Һ����2��Ϊ��ȷ�������ܽ�ɳ�����Һ��ԭ��С���ͬѧ���������̽����
������⣺����Ϊʲô���ܽ��ɳ�����Һ��
�������ϣ�̼���������ᣬ̼�����[Ca��HCO3��2]����ˮ��
��������裺����Һ�����ԣ��ڷ�Ӧ������̼����ƣ�
ʵ������ۣ�
| ʵ����� | ʵ������ | ʵ����� |
| ʵ���һСƬpH��ֽ����һ��ɾ��IJ���Ƭ�ϣ��� ������ պȡ�����ܽ�ɳ������Һմ����ֽ�ϣ�����ֽ���ֵ���ɫ�����ɫ�����գ� |
��ñ���Һ��pH=8 | ����� ������ ��������������������� |
| ʵ���ȡ�����ܽ�ɳ������Һ����һ֧�Թ��У����� ϡ���ᣨ���ᣩ �� |
��������� | ��Ӧ�Ļ�ѧ����ʽΪ�� Ca��HCO3��2+2HCl=CaCl2+2H2O+2CO2�� ������ڳ����� |
��������һ�������¿�ת��Ϊ������
����������1�����ݶ�����̼�����ʯ��ˮ��Ӧ��ʹ����ʯ��ˮ[��Ҫ�ɷ�Ca��OH��2]����ǵ������ж��Թ��ڵ������ݴ�д����Ӧʽ��
��2������ʵ�����������һ��̽�����ɸ��ݶ�����̼�����ʡ�Ca��HCO3��2������ˮ����Һ�����ȵIJⶨ�������з�������⣬����ʱ��Ҫ�����Ŀ��Ҫ����Ҫ��ϵ��ѧϰ��֪ʶ�����������������Ƶ����ٻش�
��2������ʵ�����������һ��̽�����ɸ��ݶ�����̼�����ʡ�Ca��HCO3��2������ˮ����Һ�����ȵIJⶨ�������з�������⣬����ʱ��Ҫ�����Ŀ��Ҫ����Ҫ��ϵ��ѧϰ��֪ʶ�����������������Ƶ����ٻش�
����⣺
��1��ʯ��ˮ����Ҫ�ɷ����������ƣ�ͨ�������̼������̼��ƺ�ˮ��̼�����������ˮ�İ�ɫ���壬���Կɿ����Թ����а�ɫ�������䷴Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��2��ʵ������ۣ�
ʵ���Ҫ��֤�������Һ�����ԣ�����ʯ���Լ���PH��ֽ�ⶨ����ʵ������֪pH=8��������֪ʵ���������PH��ֽ��������������ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ֱ�ɫ�ͱ���ɫ���Ƚϣ����ɵó���Һ��PHֵ��pH=8��˵����Һ�ʼ��ԣ��ʼ���ٲ�������
ʵ���Ҫ��֤����ڣ������С�̼�����[Ca��HCO3��2]����ˮ�������Ʋ���̼���������̼����ƣ����к���̼������Ϳ���ϡ���������飬�������ϡ���ᣬ�����ж�����̼����ų����ʿ�д����ѧ����ʽΪ��2HCl+Ca��HCO3��2=CaCl2+2H2O+2CO2����
��˼�뽻��������̽�����̣����ǿ��Եõ����ྭ�飺��Ҫ���ӹ۲�����Ҫ��������ʣ������̽������ֱ�ӵó���ѧ���ۣ�����������һ�������¿���ת��Ϊ�����
�ʴ�Ϊ��
��1�������ܿ������ݣ�ʯ��ˮ����ǣ�CO2+Ca��OH��2�TCaCO3��+H2O��
��2��ʵ�������
��˼�뽻������������һ�������¿�ת��Ϊ�����
��1��ʯ��ˮ����Ҫ�ɷ����������ƣ�ͨ�������̼������̼��ƺ�ˮ��̼�����������ˮ�İ�ɫ���壬���Կɿ����Թ����а�ɫ�������䷴Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��2��ʵ������ۣ�
ʵ���Ҫ��֤�������Һ�����ԣ�����ʯ���Լ���PH��ֽ�ⶨ����ʵ������֪pH=8��������֪ʵ���������PH��ֽ��������������ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ֱ�ɫ�ͱ���ɫ���Ƚϣ����ɵó���Һ��PHֵ��pH=8��˵����Һ�ʼ��ԣ��ʼ���ٲ�������
ʵ���Ҫ��֤����ڣ������С�̼�����[Ca��HCO3��2]����ˮ�������Ʋ���̼���������̼����ƣ����к���̼������Ϳ���ϡ���������飬�������ϡ���ᣬ�����ж�����̼����ų����ʿ�д����ѧ����ʽΪ��2HCl+Ca��HCO3��2=CaCl2+2H2O+2CO2����
��˼�뽻��������̽�����̣����ǿ��Եõ����ྭ�飺��Ҫ���ӹ۲�����Ҫ��������ʣ������̽������ֱ�ӵó���ѧ���ۣ�����������һ�������¿���ת��Ϊ�����
�ʴ�Ϊ��
��1�������ܿ������ݣ�ʯ��ˮ����ǣ�CO2+Ca��OH��2�TCaCO3��+H2O��
��2��ʵ�������
| ʵ����� | ʵ������ | ʵ����� |
| ʵ���һСƬpH��ֽ����һ��ɾ��IJ���Ƭ�ϣ��ò�����պȡ�����ܽ�ɳ������Һմ����ֽ�ϣ�����ֽ���ֵ���ɫ�����ɫ�����գ� | ��ñ���Һ ��pH=8 |
����ٲ������� ������������������� |
| ʵ���ȡ�����ܽ�ɳ������Һ�� ��һ֧�Թ��У�����ϡ���ᣨ���ᣩ�� |
��������� | ��Ӧ�Ļ�ѧ����ʽΪ�� Ca��HCO3��2+2HCl=CaCl2+2H2O+2CO2���� ����ڳ����� |
������̽������������һ����İ���ȣ�����������ݻ���ѧ�������ݣ�ֻ��Ҫ�������õ����龳�У�����õ���Ŀ��Ҫ���£�Ҫ����Ŀͨ�����飬�ҳ�������Ϣ���ڽ��к������Ƶ����ƣ�����������⣬̽�����ܿ���ѧ����˼ά��ʵ��������

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
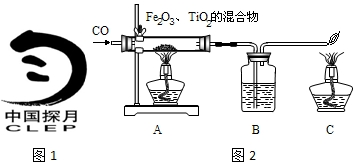
 ���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮
���϶�һ�š��ijɹ����䣬ʹȫ�����������裮��ͬѧ���Ķ����к��켼���еĻ�ѧ���IJ��ش��й����⣮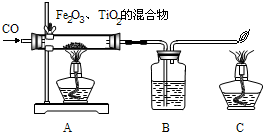
 D��ԭ�ӽṹʾ��ͼΪ
D��ԭ�ӽṹʾ��ͼΪ