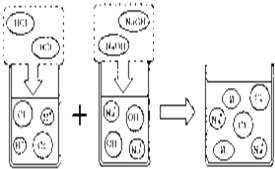
��Ŀ����
�����������ճ������г��õĸ�����ϴ����ѡ���ʵ�����Ʒ���Եõ����õ���ϴЧ����
| ���� | ϴ�ྫ | ����� | ¯������ | ���ձ�ը�� | Ư�� |
| ��Ʒ��ʽ |  |  |  |  |  |
| ��Ч�ɷ� ���� | ��ϴ���� | ���� | �������� | ��̼���� | ���� |

��1��ʹ��ϴ�ྫ��ϴ�;��ϵ����ۣ�������Ϊ������______�Ĺ��ܣ�
��2���������ʿ���ʹ�ý������ϴ����______������ĸ��ţ���
a�����⡡����b�����ա��� c��ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ��
��3��ȡ����һ������¯���������μӼ��η�̪��Һ����Һ��______ɫ�������������¯��������ϣ����Է�����ͼ��ʾ�Ļ�ѧ��Ӧ��ͼ1��a��������Ϊ______��
��4�������ձ�ը�Ρ�����ˮ�������Na2CO3��H2O2������ը������ˮ���ټ��������Ľ���飬�����Ļ�ѧ��Ӧ����ʽΪ______��
��5����ѧС�鷢��һ����װ�����Ư�ۣ�ͬѧ�Ƕ�Ư����Ư�������Ƿ�ʧЧ���������ʣ�������Ч�ɷ���ȫ��ʧʱ����Ư�۾���ȫʧЧ��������ʧʱ����Ϊ����ʧЧ����
��������
��Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2��Ca��OH��2������Ч�ɷ���Ca��ClO��2��
��Ca��ClO��2������ˮ��Ư��ԭ���ǣ����ڿ����з�����Ӧ��Ca��ClO��2+H2O+CO2=CaCO3��+2HclO��
��HClO���ȶ����ֽ�����HCl��һ�ֳ����ĵ������壮
��CaCl2��ˮ��Һ�����ԣ�HClO��ˮ��Һ�����ԣ�
��HClO�ܿ�ʹ��ɫ���ʣ��磺Ʒ����Һ����ɫ��
ʵ��̽����
�±���̽��ijƯ���Ƿ���ȫʧЧ��ʵ�飬����ݱ��н��ۣ�������գ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ������Ư����Ʒ����ˮ�У�ͨ��������CO2���壬 ______�� | ______ | Ư�۵�Ư����������ȫʧЧ |
ͬѧ�Ƕ�ij��ȫʧЧ���Ư�۵ijɷֺܸ���Ȥ��������һ��̽��������ʦ�İ����£�ͬѧ�ǶԸ�ʧЧ���Ư�۳ɷֽ��в��룺
����һ��CaCl2��CaCO3���������______��Ȼ�����ʵ��̽����
| ʵ����� | ʵ������ | ʵ����� |
| ����Ʒ����ˮ�У�����ܽ����ˣ�______�� | ______ | ����һ���� |
��HClO�ֽ�ʱ������HCl�⣬���ɵ���һ�ֳ���������______��
�ڲ����еijɷ�CaCO3����Ư�۵���Ч�ɷ��ڿ����з�����Ӧ�����⣬����������Դ�������û�ѧ����ʽ��ʾ______��
�������
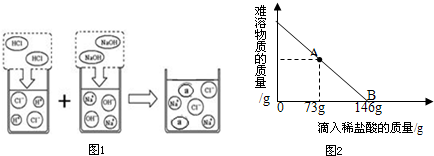
ȡ����Ư��31.1g��һ�ձ��У������м���131.7gˮ��ʹƯ���еĿ�������ȫ�ܽ⣮Ȼ������������μ���������������Ϊ10%��ϡ���ᣬ�ձ������ܹ������ʵ�������������ϡ�����������ϵ������ͼ2��ʾ�����������ش��������⣺
�ٵ�����10%��ϡ������ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ��______��
�ڵ�����10%��ϡ����146gʱ����B�㣩����ͨ�����㣬���ʱ�ձ������ò�������Һ�����ʵ�����������
�⣺��1��ϴ�ྫ���黯���ܣ�����ϴ�;��ϵ����ۣ�
��2������������ԣ������ᷴӦ�����ʾ���������ϴ��ѡa c��
��3��¯�������Լ��ԣ���ʹ��̪��Һ���ɫ��������к��������ӣ�¯�������к������������ӣ������ʻ�ϣ������������������ӽ�ϳ�ˮ���ӣ�
��4�������ձ�ը�Ρ�����ˮ�������Na2CO3��H2O2������ը������ˮ���ټ��������Ľ���飬̼�����������е����ᷢ����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2��
��5��ʵ��̽��������Ca��ClO��2+H2O+CO2=CaCO3+2HClO��HClO��ʹ��ɫ������Ʒ����Һ��ɫ�����Կ�������Ʒ����ɫ��ȥ���ʵ�飮����Ʒ����Һ�������ɫ����ȥ��˵��Ư��ʧЧ��
����̽�����������������Ŀ��Ϣ��֪��Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2��Ca��OH��2��Ca��ClO��2+H2O+CO2=CaCO3+2HClO�����ǿ����ж�CaCl2��CaCO3��Ca��OH��2ȡ��Һ���Թ��У������е�����ɫ��̪��Һ��ͨ��CO2����Һ�����Ա仯
�������ۣ�HClO���ȶ����ֽ�����HCl��һ�ֳ�������ɫ��ζ���壬���������غ㶨�ɿ�֪����һ����������
���������������̼��ӦҲ������̼��ƣ���ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O
�����о����ټ������ᣬ̼��ƺ����ᷴӦ�����Ȼ��ƣ����A��ʱ��Һ�е��������Ȼ��ƣ�
�ڸ���B����ȫ��Ӧ��ʱ�����������������������Ȼ��ƺͲμӷ�Ӧ��̼��Ƶ�������
�����ɵ��Ȼ�������Ϊx���μӷ�Ӧ��̼�������Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111
y 146g��10% x
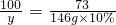
y=20g
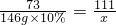
x=22.2g
B����Һ�������Ȼ��Ƶ�����Ϊ��22.2g+31.1g-20g=33.3g
��Һ����Ϊ��146g+31.1g+131.7g-8.8g=300g
������������=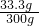 ��100%=11.1%
��100%=11.1%
����Һ��������������Ϊ11.1%
�ʴ�Ϊ����1���黯 ��2��a c ��3���죻ˮ����
��4��Na2CO3+2HCl=2NaCl+H2O+CO2��
��5����ʵ��̽��������Ʒ����Һ��Ʒ����Һ����ɫ
������̽�����������CaCl2��CaCO3��Ca��OH��2
ȡ��Һ���Թ��У������е�����ɫ��̪��Һ��ͨ��CO2����Һ�����Ա仯
���������ۡ�
����������O2�� ��Ca��OH��2+CO2=CaCO3��+H2O
�������о�
��CaCl2
��11.1%
��������1������ϴ�����ȥ��ԭ��������
��2�����ݽ����ijɷּ����ʵ����ʷ�����
��3���������ӷ�Ӧ��ʵ�ʼ����ʵĴ�����ʽ�����жϣ�
��4������̼���ƺ����ᷴӦ��ԭ������ѧ����ʽ��дҪ������ɣ�
��5���������ۣ����������غ㶨�ɿ�֪��HClO�ֽ������HCl�⣬���ɵ���һ�ֳ���������������
ʵ��̽����������Ŀ��Ϣ��֪��Ca��ClO��2������ˮ��Ư��ԭ���ǣ����ڿ����з�����ӦCa��ClO��2+H2O+CO2=CaCO3+2HClO��HClO��ʹ��ɫ������Ʒ����Һ��ɫ�����ԾͿ�����Ʒ����飻
�������������Ŀ��Ϣ��֪��Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2��Ca��OH��2�����ǿ����ж�CaCl2��CaCO3��Ca��OH��2������������ɣ����ǿ��Եõ�Ca��OH��2�Ͷ�����̼����̼��ƣ�
����̽�������ݻ�ѧ����ʽ����֪�Ȼ����������������ɵ��Ȼ��ƺͲμӷ�Ӧ��̼����������Ӷ������Һ��CaCl2������Ϊ22.2g+31.1g-20g=33.3g ��Һ������Ϊ146g+31.1g+131.7g-8.8g=300g ���������������
������������Ҫ���ճ������г��õ�����Ϊ�زģ�������ѧ�����û�ѧ֪ʶ���ʵ�������������
��2������������ԣ������ᷴӦ�����ʾ���������ϴ��ѡa c��
��3��¯�������Լ��ԣ���ʹ��̪��Һ���ɫ��������к��������ӣ�¯�������к������������ӣ������ʻ�ϣ������������������ӽ�ϳ�ˮ���ӣ�
��4�������ձ�ը�Ρ�����ˮ�������Na2CO3��H2O2������ը������ˮ���ټ��������Ľ���飬̼�����������е����ᷢ����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2��
��5��ʵ��̽��������Ca��ClO��2+H2O+CO2=CaCO3+2HClO��HClO��ʹ��ɫ������Ʒ����Һ��ɫ�����Կ�������Ʒ����ɫ��ȥ���ʵ�飮����Ʒ����Һ�������ɫ����ȥ��˵��Ư��ʧЧ��
����̽�����������������Ŀ��Ϣ��֪��Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2��Ca��OH��2��Ca��ClO��2+H2O+CO2=CaCO3+2HClO�����ǿ����ж�CaCl2��CaCO3��Ca��OH��2ȡ��Һ���Թ��У������е�����ɫ��̪��Һ��ͨ��CO2����Һ�����Ա仯
�������ۣ�HClO���ȶ����ֽ�����HCl��һ�ֳ�������ɫ��ζ���壬���������غ㶨�ɿ�֪����һ����������
���������������̼��ӦҲ������̼��ƣ���ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O
�����о����ټ������ᣬ̼��ƺ����ᷴӦ�����Ȼ��ƣ����A��ʱ��Һ�е��������Ȼ��ƣ�
�ڸ���B����ȫ��Ӧ��ʱ�����������������������Ȼ��ƺͲμӷ�Ӧ��̼��Ƶ�������
�����ɵ��Ȼ�������Ϊx���μӷ�Ӧ��̼�������Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111
y 146g��10% x
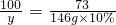
y=20g
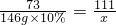
x=22.2g
B����Һ�������Ȼ��Ƶ�����Ϊ��22.2g+31.1g-20g=33.3g
��Һ����Ϊ��146g+31.1g+131.7g-8.8g=300g
������������=
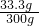 ��100%=11.1%
��100%=11.1% ����Һ��������������Ϊ11.1%
�ʴ�Ϊ����1���黯 ��2��a c ��3���죻ˮ����
��4��Na2CO3+2HCl=2NaCl+H2O+CO2��
��5����ʵ��̽��������Ʒ����Һ��Ʒ����Һ����ɫ
������̽�����������CaCl2��CaCO3��Ca��OH��2
ȡ��Һ���Թ��У������е�����ɫ��̪��Һ��ͨ��CO2����Һ�����Ա仯
���������ۡ�
����������O2�� ��Ca��OH��2+CO2=CaCO3��+H2O
�������о�
��CaCl2
��11.1%
��������1������ϴ�����ȥ��ԭ��������
��2�����ݽ����ijɷּ����ʵ����ʷ�����
��3���������ӷ�Ӧ��ʵ�ʼ����ʵĴ�����ʽ�����жϣ�
��4������̼���ƺ����ᷴӦ��ԭ������ѧ����ʽ��дҪ������ɣ�
��5���������ۣ����������غ㶨�ɿ�֪��HClO�ֽ������HCl�⣬���ɵ���һ�ֳ���������������
ʵ��̽����������Ŀ��Ϣ��֪��Ca��ClO��2������ˮ��Ư��ԭ���ǣ����ڿ����з�����ӦCa��ClO��2+H2O+CO2=CaCO3+2HClO��HClO��ʹ��ɫ������Ʒ����Һ��ɫ�����ԾͿ�����Ʒ����飻
�������������Ŀ��Ϣ��֪��Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2��Ca��OH��2�����ǿ����ж�CaCl2��CaCO3��Ca��OH��2������������ɣ����ǿ��Եõ�Ca��OH��2�Ͷ�����̼����̼��ƣ�
����̽�������ݻ�ѧ����ʽ����֪�Ȼ����������������ɵ��Ȼ��ƺͲμӷ�Ӧ��̼����������Ӷ������Һ��CaCl2������Ϊ22.2g+31.1g-20g=33.3g ��Һ������Ϊ146g+31.1g+131.7g-8.8g=300g ���������������
������������Ҫ���ճ������г��õ�����Ϊ�زģ�������ѧ�����û�ѧ֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ




















 ��1������ʹ��ϴ������ϴ�;��ϵ����ۣ�������Ϊ������
��1������ʹ��ϴ������ϴ�;��ϵ����ۣ�������Ϊ������