��Ŀ����
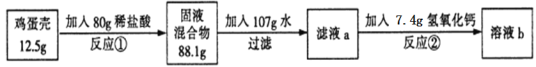
����Ŀ�������ǵ���Ҫ�ɷ���̼���(�����ɷֲ�����ˮҲ�����ᷴӦ)����ѧ��ȤС��Ϊ�˲ⶨ��������̼��Ƶĺ�����������ʵ�顣��Ӧ�ٽ�������������պ÷�Ӧ��һ�룬��Ӧ��ǡ����ȫ��Ӧ��
��ش��������⣺
��1����Ӧ�ٵĻ�ѧ����ʽΪ________��
��2��������֪�����г����̼�������(X)�ı���ʽ______________��
��3���ü�������̼��Ƶ���������Ϊ_______��
��4����Һb�����ʵ���������Ϊ_______��
��5����36.5����Ũ��������80g����ϡ�������ˮ������Ϊ_______��
���𰸡�CaCO3 + 2HCl = CaCl2 + H2O + CO2�� 100/44=X/4.4g 80% 11.1% 40g
��������
��1����Ӧ�ٵĻ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
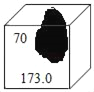
��2����̼��Ƶ�����Ϊx����Ӧ���ɶ�����̼������=12.5g+80g-88.1g=4.4g��������
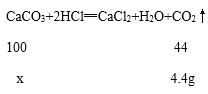
![]()
��3������2������ʽ�ɽ��x=10g���ü�������̼��Ƶ���������=![]() ��100%=80%��
��100%=80%��
��4����Һb������=10g+80g+107g+7.4g-4.4g=200g����Һb�����ʵ���������=![]() ��100%=11.1%��
��100%=11.1%��
��5������Ҫ36.5%��Ũ���������Ϊw����w��36.5%=7.3g+7.3g�����w=40g����36.5%��Ũ��������80g����ϡ�������ˮ������=80g-40g=40g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�