��Ŀ����
����Ŀ��Ư�۵���Ҫ�ɷ�Ϊ������� [Ca(ClO)2] ���Ȼ��ƣ���������Ư�����ֿ�������������Ư������Ч�ɷ��� [Ca(ClO)2] ��Ư��ԭ���ǣ�Ca(ClO)2�ڿ����з�����Ӧ��Ca(ClO)2+ CO2+ H2O��CaCO3��+ 2HClO��HClO��һ�����Ա����������ᣬ���ȶ�������Ư���ԣ���ʹƷ�����ɫ������ɫ��
��1��HClO�ڳ����·ֽ⣬��ӦΪ2HClO��2HCl+ O2����ijƿHClO��Һ����һ��ʱ�����Һ��pH �������С�����䡱����
��2����Ư���еμ����ᣬ�ܼӿ�Ư�����ʡ�д��Ư���м������ᷴӦ�Ļ�ѧ����ʽ��
��3����һ����ʱ����õ�Ư�ۣ���֪����Ư���Ƿ���ʣ�ijͬѧ���������̽��ʵ�顣
��������⡿��ʱ����õ�Ư���Ƿ���ʣ�
���� �롿
����1����Ư��δ���ʣ�����ɷ�ΪCaCl2��Ca(ClO)2��
����2����Ư�۲��ֱ��ʣ�����ɷ�Ϊ��
����3����Ư��ȫ�����ʣ�����ɷ�ΪCaCl2��CaCO3��
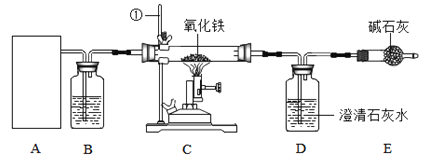
��4����ʵ��̽������ѡ�Լ������������ᡢʯ��ˮ��Ʒ����Һ���Թܡ������ܵĵ�������
ʵ����� | ʵ������ | ʵ����� |
��ȡ������Ʒ���Թ��У��� | ���������������ʹʯ��ˮ����ǡ� | ����2���� |
����������Ӧ���Թ��м������� | �� |
��5������ʵ����HCl��CaCl2�Ļ����Һ��Ϊ�˷��������Һ��HCl��CaCl2���������������������ʵ�鷽����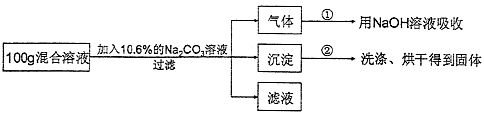
��ʵ�����ݡ�����ʵ����100g�����Һ��mg���ʵ���������Ϊ10.6%��Na2CO3��Һǡ����ȫ��Ӧ����¼������ʵ�����ݣ��ڢ��飬����������ȫ��NaOH��Һ��������4.4g���ڢ��飬������ȫ�����ˡ�ϴ�ӡ���ɺ�õ����������Ϊ10g��
����ʵ����Ƽ��й����ݽ��з�������㣺
m= ��
��6������ʵ����100g�����Һ��mg���ʵ���������Ϊ10.6%��Na2CO3��Һǡ����ȫ��Ӧ�������˺����á���Һ�������ʵ���������Ϊ���٣����������ǹ����е���ʧ��������������С�����һλ������
���𰸡�
��1����С
��2��Ca(ClO)2+2HCl=CaCl2+2HClO
��3��CaCl2 Ca(ClO)2 CaCO3
��4��������������,���ϴ����ܵĵ�����,�ѵ��ܲ�����һ�Թ����ʯ��ˮ��,Ʒ����Һ,Ʒ����Һ��ɫ
��5��200g
��6��8.2��
����������1��HClO�ڳ����·ֽ⣬��ӦΪ2HClO��2HCl+ O2������Ӧ�������������䣬����Һ���������٣�������ǿ����Һ��pH��С����2��Ư���м������ᷴӦ�Ļ�ѧ����ʽCa(ClO)2 + 2HCl = CaCl2 + 2HClO��(3). Ư�۵���Ҫ�ɷ�Ϊ������� [Ca(ClO)2] ���Ȼ��ƣ�Ư��ԭ���ǣ�Ca(ClO)2�ڿ����з�����Ӧ��Ca(ClO)2+ CO2+ H2O��CaCO3��+ 2HClO��Ư�۲��ֱ��ʣ�����ɷ�ΪCaCl2 Ca(ClO)2 CaCO3 (4). �����������ᣬ���ϴ����ܵĵ��������ѵ��ܲ�����һ�Թ����ʯ��ˮ�У����������������ʹʯ��ˮ����ǣ�֤����������̼��ƣ�. �������д�����ƣ���ˮ���ɴ����ᣬ��Ư�����ã�������Ӧ�����Һ�м���Ʒ����Һ ��Ʒ����Һ��ɫ��֤���������д�����ơ� (5��6). �������غ㶨�ɿ�֪��Ӧǰ�����ʵ����������䣬��ǡ����ȫ��Ӧ��������̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCl= | 2NaCl+H2O+ | CO2�� |
106 | 117 | 44 |
x | y | 4.4g |
106/x=44/4.4g x=10.6g
117/y=44/4.4g y=11.7g
̼������Һ������Ϊ��10.6g��10.6%=100g��
�����Ȼ��Ʒ�Ӧ��̼���Ƶ�����Ϊm,�����Ȼ��Ƶ�����Ϊn��
CaCl2+ | Na2CO3= | CaCO3��+ | 2NaCl |
106 | 100 | 117 | |
m | n |
100/10g=106/m m=10.6g
100/10=117/n n=11.7g
���Ȼ��Ʒ�Ӧ��̼������Һ����Ϊ��10.6g��10.6%=100g��
����m=100g+100g=200g
��Һ�����ʵ���������Ϊ�� ![]() ��8.2��
��8.2��
�ʴ�Ϊ����С��Ca(ClO)2+2HCl=CaCl2+2HClO��CaCl2�� Ca(ClO)2�� CaCO3�������������ᣬ���ϴ����ܵĵ��������ѵ��ܲ�����һ�Թ����ʯ��ˮ�С�Ʒ����Һ��Ʒ����Һ��ɫ��200g��8.2����
��ѧ����ʽ����д������֪��Ϣд�����ɣ����������������غ㶨�ɣ��μӷ�Ӧ�������������������ɵ�����������������֮�ȵ�����Է����������Ի�ѧ������֮�ȡ�