��Ŀ����
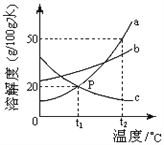
����Ŀ����̼�����ܡ��������ҹ���һ��������ߣ���ν����̼����������ָ�������ճ������о����ܼ����������ģ�����![]() �ŷţ�ע���ˮ�����͡���������������һ��̬�ȣ�������һ�����Σ�����һ��ȫ�µ��������������������������̼������������ǣ� ��
�ŷţ�ע���ˮ�����͡���������������һ��̬�ȣ�������һ�����Σ�����һ��ȫ�µ��������������������������̼������������ǣ� ��
A.�������Ҫ����Ʒ���ٴ����˲��ʵķ�װ
B.�ܾ�ʹ��һ����ľ�꣬�õ��Ӻؿ�����ֽ�ʺؿ�
C.������������������������
D.�ᳫʹ��һ�������ϴ�����
���𰸡�D
��������
A���������Ҫ����Ʒ���ٴ����˲��ʵķ�װ���Լ��ٻ�����Ⱦ������ȷ�����������⣻
B���ܾ�ʹ��һ����ľ�꣬�õ��Ӻؿ�����ֽ�ʺؿ����Լ�����Ŀɭ�ֵĿ������ʿ��Լ��ٻ�����Ⱦ������ȷ�����������⣻
C�����������г���������������п��Լ��ٻ�ʯȼ�ϵ�ȼ�գ����Լ��ٻ�����Ⱦ������ȷ�����������⣻
D���ᳫʹ��һ�������ϴ��������ɰ�ɫ��Ⱦ���ʴ��������⡣��ѡD��

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�����Ŀ������������������������й㷺��Ӧ�á�ʵ��С��ͬѧ�������ϵ�֪�����ᣨH2C2O4����ʹ��������ĸ��������Һ��ɫ������ͬ��������ɫʱ�䲻ͬ������Ӧ�����ʲ�ͬ��С��ͬѧ����������̽����
��������⣩Ӱ��÷�Ӧ��Ӧ���ʵ���������Щ��
���������룩Ӱ��÷�Ӧ��Ӧ���ʵ��������¶ȡ������������Ũ�ȵȡ�
���������ϣ������̣�MnSO4�������÷�Ӧ�Ĵ�����
������ʵ�飩ȡA��B��C��D 4֧�Թܣ�ÿ֧�Թ��зֱ����4mL 0.08% ��KMnO4 ��Һ��0.4 mL �����ᡢ1mL 0.09% �� H2C2O4 ��Һ��

��ʵ���¼��
��� | ��������Ũ�� | �¶� | ���� | ���������ȫ��ɫʱ�� |
A | 98% | ���� | �� | 72s |
B | 65% | ���� | �� | 129s |
C | 65% | 50�� | �� | 16s |
D | 65% | ���� | MnSO4 | 112s |
����������ۣ�
��1������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽ���£����ں����ϲ�ȫ����ʽ��
2KMnO4 + 5H2C2O4 + 3H2SO4 == K2SO4 + 2MnSO4 + 10_______+ 8H2O
��2��4֧�Թ�����Ϊ����ʵ�����_______������ţ���ͬ����
��3�����ʵ��A��B��Ŀ���� ______________________________��
��4���Ա�ʵ��B��C�ɵó��Ľ�����_____________________________��
��5��̽�������Ը÷�Ӧ��Ӧ����Ӱ���ʵ����____________________________��
����˼�뽻����
��6��Ӱ��÷�Ӧ��Ӧ���ʵ����س��¶ȡ������������Ũ���⣬��������_______��
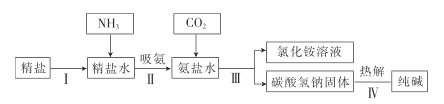
����Ŀ����ҵ�ϳ�������˫�����ȥ�����е�![]() ����ҵ����ʾ��ͼ����.
����ҵ����ʾ��ͼ����.
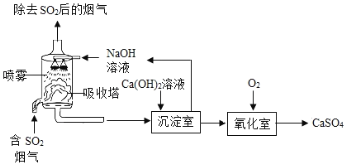
(1)��������,��NaOH��Һ����![]() ����ѧ��Ӧ����ʽ��___.NaOH��Һ�����״�ܳ������SO2��ԭ����_____
����ѧ��Ӧ����ʽ��___.NaOH��Һ�����״�ܳ������SO2��ԭ����_____
(2)�������У���ѧ��Ӧ����ʽ��_______��
(3)��֪����ԭ�ϵļ۸������ʾ.
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.90 |
������ҵ������,������ͬ����![]() ��˫������ԭ�ϳɱ�����NaOHֱ�����ո���,ԭ����___
��˫������ԭ�ϳɱ�����NaOHֱ�����ո���,ԭ����___
(4)ij��ѧ��ѧ��ȤС��Ϊ�ⶨ������Χ�Ŀ����еĶ����������Ƿ���Ϲ��ұ�,��![]() ��Χ����ͨ��һ��������(
��Χ����ͨ��һ��������(![]() )2.54mg�ĵ�ˮ��,���ⶨI2���������ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��
)2.54mg�ĵ�ˮ��,���ⶨI2���������ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��![]() ��ͨ������˵���������ŷź���Χ�����ж��������Ũ�ȼ���______��
��ͨ������˵���������ŷź���Χ�����ж��������Ũ�ȼ���______��
[������Ϣ:�ҹ������������Կ����ж��������Ũ�ȼ���涨���±���ʾ(�����ж��������Ũ���õ�λ����Ŀ������������������������ʾ)]��
Ũ�ȼ��� | ��(mg��m-3) |
һ�� | Ũ����0.15 |
���� | 0.15<Ũ����0.50 |
���� | 0.50<Ũ����0.70 |