��Ŀ����
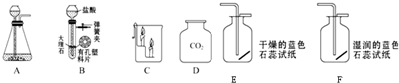
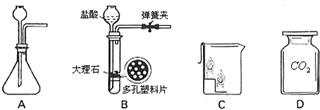
ijѧ���ڽ��ж�����̼����ȡ������ʵ��ʱ��������������о����Կα��е����巢��װ�ã���ͼA�������˸Ľ�����ͼB����

��1���Ľ���װ�õ��ŵ���
��2�����ƵõĶ�����̼����ͨ��ʢ�г���ʯ��ˮ���Թ��У��۲쵽��������
��3����D�е�CO2����C��������ʵ��ʱ���۲쵽��������a
��4��ʵ������ȡCO2�����ֱ���ʽ����ű���ʽ��

��1���Ľ���װ�õ��ŵ���
���ڿ��Ʒ�Ӧ�Ľ���
���ڿ��Ʒ�Ӧ�Ľ���
����2�����ƵõĶ�����̼����ͨ��ʢ�г���ʯ��ˮ���Թ��У��۲쵽��������
����ʯ��ˮ�����
����ʯ��ˮ�����
����3����D�е�CO2����C��������ʵ��ʱ���۲쵽��������a
��������ɵ͵�������Ϩ��
��������ɵ͵�������Ϩ��
����ʵ��˵��������̼b����ȼ��Ҳ��֧��ȼ���ܶȣ��ȿ�����
����ȼ��Ҳ��֧��ȼ���ܶȣ��ȿ�����
��4��ʵ������ȡCO2�����ֱ���ʽ����ű���ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����������1���Կα��е����巢��װ�ã�ͼA�������˸Ľ���ͼB�����������п����ϰ壬ʹ�ù���ҩƷ��Һ��ҩƷ���Է��룻�ı���װ��A��ҩƷһ�Ӵ���ֱ�ӷ�Ӧ�IJ��㣻
��2��ѧ����������̼����ķ�����
��3��̽��������̼�����ʼȲ���ȼ��Ҳ��֧��ȼ�����ܶȱȿ������ʵ�飮
��4����ȷ������д��ȡ������̼�Ļ�ѧ����ʽ��
��2��ѧ����������̼����ķ�����
��3��̽��������̼�����ʼȲ���ȼ��Ҳ��֧��ȼ�����ܶȱȿ������ʵ�飮
��4����ȷ������д��ȡ������̼�Ļ�ѧ����ʽ��
����⣺��1��װ�øĽ�����пװ���ѹ�����Һ����뿪�������رյ���ʱ��װ����ѹǿ���߶���Һ��ѹ�س���©����ʹ��Һ�����������Ӵ���Ӧֹͣ�������ܣ�װ����ѹǿ��С������©����Һ����������װ�ã�Һ�������Ӵ�����Ӧ������
��2�����������̼����ķ��������ƵõĶ�����̼����ͨ��ʢ�г���ʯ��ˮ���Թ��У��۲쵽�������ǣ�����ʯ��ˮ����ǣ�
��3����D�е�CO2����C��������ʵ��ʱ���������������ձ����������룬����Ѹ�ٵ���ʱ������Ϩ����Ⱥ�������ԣ�����ʵ��ʧ�ܣ���ȷ�Ĺ۲���������������ɵ͵�������Ϩ�������˶�����̼�Ȳ���ȼ��Ҳ��֧��ȼ�����ܶȱȿ���������ʣ�
��4����ȷ��д��ȡ������̼�Ļ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
�ʴ�Ϊ��
��1�����ڿ��Ʒ�Ӧ�Ľ��У�
��2������ʯ��ˮ����ǣ�
��3����������ɵ͵�������Ϩ�𣻲���ȼ��Ҳ��֧��ȼ���ܶȣ��ȿ�����
��4��CaCO3+2HCl�TCaCl2+H2O+CO2����
��2�����������̼����ķ��������ƵõĶ�����̼����ͨ��ʢ�г���ʯ��ˮ���Թ��У��۲쵽�������ǣ�����ʯ��ˮ����ǣ�
��3����D�е�CO2����C��������ʵ��ʱ���������������ձ����������룬����Ѹ�ٵ���ʱ������Ϩ����Ⱥ�������ԣ�����ʵ��ʧ�ܣ���ȷ�Ĺ۲���������������ɵ͵�������Ϩ�������˶�����̼�Ȳ���ȼ��Ҳ��֧��ȼ�����ܶȱȿ���������ʣ�
��4����ȷ��д��ȡ������̼�Ļ�ѧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
�ʴ�Ϊ��
��1�����ڿ��Ʒ�Ӧ�Ľ��У�
��2������ʯ��ˮ����ǣ�
��3����������ɵ͵�������Ϩ�𣻲���ȼ��Ҳ��֧��ȼ���ܶȣ��ȿ�����
��4��CaCO3+2HCl�TCaCl2+H2O+CO2����
����������̽����ʵ������ȡ������̼�����װ�ü��Ľ�����ŵ㣬�����˶�����̼����ļ��鷽������д�䷴Ӧ�Ļ�ѧ����ʽ����̽���˶�����̼�Ȳ���ȼ��Ҳ��֧��ȼ�����ܶȱȿ���������ʵ�ʵ�飮

��ϰ��ϵ�д�
�����Ŀ