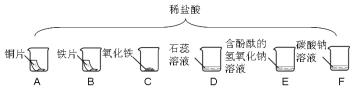
��Ŀ����
����Ŀ��ij��ѧʵ��С���ͬѧ����50g������������Ϊ3%�Ȼ�����Һ���ش����⣺
��1������������������Ϊ6%�Ȼ�����Һ���ܶ�ԼΪ1.04g/cm3����ˮϡ�����ƣ���ȡ����Ҫ��6%���Ȼ�����Һʱ��Ӧѡ��________���20mL����50mL����100mL����������Ͳ��
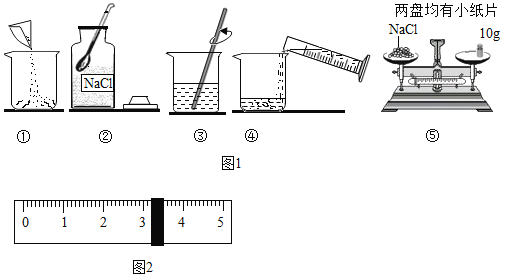
��2�������Ȼ��ƹ����ˮ���Ƹ���Һ������������ͼ����ȷ����˳����______������ĸ����
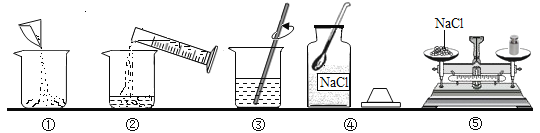
A�ܢݢ٢ڢ�
B�٢ڢۢܢ�
C�ۢܢ٢ڢ�
D�ڢ٢ܢۢ�
��3�����ѵ���ƽ���������ƽ��ȡ�Ȼ��ƹ���ʱ������ָ��ƫ�ң��������IJ�����________��
��4������ȡˮ�Ĺ����У������Ӷ�ȡ��Ͳ��ˮ�������������������ȷ�����������Ƶ��Ȼ�����Һ������������_______���ƫ�����䡱��ƫС������
���𰸡�50 mL A ����ҩƷ ƫС
��������
��1������ϡ��ǰ�����ʵ��������䣬������Ҫ��6%���Ȼ�����Һ������Ϊx��50g��3%=6%x��x=25g�������ԼΪ��![]() ��Ӧѡȡ��������������������Ͳ����������С����Ӧѡ��50mL��̵���Ͳ��
��Ӧѡȡ��������������������Ͳ����������С����Ӧѡ��50mL��̵���Ͳ��
��2�������Ȼ��ƹ����ˮ���Ƹ���Һ����������Ϊ�����㣨����������Ȼ��ƺ�ˮ��������������������������Ȼ�������������ȡ����ȡ�����ˮ����������ܽ⡢װƿ����ǩ������ȷ��˳���ǣ��ܢݢ٢ڢۡ�
��ѡA��
��3����������ƽ������Ʒʱ����ӡ��������롱��ԭ����ָ��ƫ�ң�˵���Ȼ��Ƶ�����ƫ�٣���Ӧ����ҩƷ��
��4�����Ӷ��飬��ȡ��ֵС��ʵ����ֵ����ʹ��ȡˮ�����ƫ����Һ����ƫ��������������ƫС��

����Ŀ���ᡢ��ε��ܽ��Ա���ѧϰ��ѧ����Ҫ����֮һ���±��Dz����ᡢ�����ˮ�е��ܽ��ԣ�20��C������ش��������⡣
������ ������ | OH- | NO3- | CO32- | Cl- |
K+ | �� | �� | �� | �� |
Ba2+ | �� | �� | �� | �� |
Cu2+ | �� | �� | �� | �� |
��1�����������������γɲ����Լ�Ļ�ѧʽΪ________�� �γɸ��Ϸ��ϵĻ�ѧʽΪ_____ ��
��2��KOH��Һ��Ba��NO3��2��Һ_______������������������������Ӧ��������_______��
��3�����и���������ˮ��Һ���ܴ����������_______________ ��
A. OH-H+Ba2+NO![]() B. OH-Ba2+K+Cl-
B. OH-Ba2+K+Cl-
C. CO![]() K+Ba2+Cl-D. CO
K+Ba2+Cl-D. CO![]() Cu2+NO
Cu2+NO![]() K+
K+