��Ŀ����
��ȡ3��73��ijŨ�ȵ����ᣬ�ֱ����ʢ��NaHCO3��KHCO3��ɵ�ͬһ�ֻ�����У����мס��ҡ�������ʵ�飬����������ݣ�������CO2��ˮ�е��ܽ⣩��
�ش��������⣺
��1�������������������Ϊ______��
��2���������NaHCO3��KHCO3��������Ϊ______��
����֪��������A��NaHCO3��KHCO3��MgCO3��CaCO3���������е����ֻ�϶��ɣ�ͨ������������ش��������⣮
��1��ȡA�����������ᷴӦ����A������Ϊ��ֵʱ����������ɳɷֵĺ�����α仯�����ɵ����������Ϊ��ֵ����A�Ŀ�������ǣ����Բ�������Ҳ�ɲ��䣩
��2����A��MgCO3��CaCO3��ɵĻ���ȡ10.0�˵�A�����������ᷴӦ���������������x��Χ�Ƕ��٣�
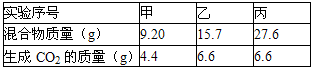
| ʵ����� | �� | �� | �� |
| �����������g�� | 9.20 | 15.7 | 27.6 |
| ����CO2��������g�� | 4.4 | 6.6 | 6.6 |
��1�������������������Ϊ______��
��2���������NaHCO3��KHCO3��������Ϊ______��
����֪��������A��NaHCO3��KHCO3��MgCO3��CaCO3���������е����ֻ�϶��ɣ�ͨ������������ش��������⣮
��1��ȡA�����������ᷴӦ����A������Ϊ��ֵʱ����������ɳɷֵĺ�����α仯�����ɵ����������Ϊ��ֵ����A�Ŀ�������ǣ����Բ�������Ҳ�ɲ��䣩
| ������ | ������ | ������ | |
| A����� | ______ | ______ | ______ |
��
��1��NaHCO3��KHCO3�����ᷴӦ�ķ���ʽ�ֱ�Ϊ��NaHCO3+HCl�TNaCl+CO2��+H2O��KHCO3+HCl�TKCl+CO2��+H2O���ɷ���ʽ��֪��HCl��CO2���ݴ˹�ϵʽ������⣻
��73��ijŨ�ȵ����������ʵ�������x
HCl��CO2
36.5 44
x 6.6g
=
���x=5.475g
���������������������������
��100%=7.5%
�ʴ�Ϊ��7.5%��
��2���ü������ݽ��
��������̼�����Ƶ�����Ϊy����̼����ص�����Ϊ9.2g-y����̼�����ƶ�Ӧ�Ķ�����̼������Ϊz��
NaHCO3+HCl�TNaCl+CO2��+H2O
84 44
y z
=
--------------��
KHCO3+HCl�TKCl+CO2��+H2O
100 44
9.2g-y 4.4g-z
=
---------��
���ݢ٢ڽ�ã�y=4.2g����̼�����Ƶ�����Ϊ4.2g��̼����ص�����Ϊ5g����������Ϊ4.2g��5g=21��25��
�ʴ�Ϊ��21��25��
��
��1����CaCO3+2HCl�TCaCl2+CO2��+H2O��KHCO3+HCl�TKCl+CO2��+H2O����������ʽ�ɿ�����
CaCO3��KHCO3�������̼�������ȶ���100��44�����Ի����A��̼��ƺ�̼����������ȿ���Ϊ����ȣ����ɵ����嶼Ϊ��ֵ��
ͬ������MgCO3+2HCl�TMgCl2+CO2��+H2O��NaHCO3+HCl�TNaCl+CO2��+H2O����������ʽ�ɿ�����
MgCO3��KHCO3�������̼�������ȶ���84��44�����Ի����A��̼��þ��̼�����������ȿ���Ϊ����ȣ����ɵ����嶼Ϊ��ֵ��
�ʴ�Ϊ��
��2����10.0�˵�A��ֻ����̼��þ�����������������������̼������Ϊa����10.0�˵�A��ֻ����̼��ƣ������������ᷴӦ�����Ķ�����̼������Ϊb
MgCO3+2HCl�TMgCl2+CO2��+H2O
84 44
10.0g a
=
a=5.24g
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
10.0g b
=
b=4.40g
���ԣ��������������x��Χ�ǣ�4.40g��x��5.24g��
��10.0��A�����������ᷴӦ���������������x��Χ��4.40g��x��5.24g��
��1��NaHCO3��KHCO3�����ᷴӦ�ķ���ʽ�ֱ�Ϊ��NaHCO3+HCl�TNaCl+CO2��+H2O��KHCO3+HCl�TKCl+CO2��+H2O���ɷ���ʽ��֪��HCl��CO2���ݴ˹�ϵʽ������⣻
��73��ijŨ�ȵ����������ʵ�������x
HCl��CO2
36.5 44
x 6.6g
| 36.5 |
| 44 |
| x |
| 6.6g |
���x=5.475g
���������������������������
| 5.475g |
| 73g |
�ʴ�Ϊ��7.5%��
��2���ü������ݽ��
��������̼�����Ƶ�����Ϊy����̼����ص�����Ϊ9.2g-y����̼�����ƶ�Ӧ�Ķ�����̼������Ϊz��
NaHCO3+HCl�TNaCl+CO2��+H2O
84 44
y z
| 84 |
| 44 |
| y |
| z |
KHCO3+HCl�TKCl+CO2��+H2O
100 44
9.2g-y 4.4g-z
| 100 |
| 44 |
| 9.2g-y |
| 4.4g-z |
���ݢ٢ڽ�ã�y=4.2g����̼�����Ƶ�����Ϊ4.2g��̼����ص�����Ϊ5g����������Ϊ4.2g��5g=21��25��
�ʴ�Ϊ��21��25��
��
��1����CaCO3+2HCl�TCaCl2+CO2��+H2O��KHCO3+HCl�TKCl+CO2��+H2O����������ʽ�ɿ�����
CaCO3��KHCO3�������̼�������ȶ���100��44�����Ի����A��̼��ƺ�̼����������ȿ���Ϊ����ȣ����ɵ����嶼Ϊ��ֵ��
ͬ������MgCO3+2HCl�TMgCl2+CO2��+H2O��NaHCO3+HCl�TNaCl+CO2��+H2O����������ʽ�ɿ�����
MgCO3��KHCO3�������̼�������ȶ���84��44�����Ի����A��̼��þ��̼�����������ȿ���Ϊ����ȣ����ɵ����嶼Ϊ��ֵ��
�ʴ�Ϊ��
| ������ | ������ | ������ | |
| A����� | NaHCO3��MgCO3 | KHCO3��CaCO3 | �������� |
MgCO3+2HCl�TMgCl2+CO2��+H2O
84 44
10.0g a
| 84 |
| 44 |
| 10.0g |
| a |
a=5.24g
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
10.0g b
| 100 |
| 10.0g |
| 44 |
| b |
b=4.40g
���ԣ��������������x��Χ�ǣ�4.40g��x��5.24g��
��10.0��A�����������ᷴӦ���������������x��Χ��4.40g��x��5.24g��

��ϰ��ϵ�д�
�����Ŀ
��ȡ3��73��ijŨ�ȵ����ᣬ�ֱ����ʢ��NaHCO3��KHCO3��ɵ�ͬһ�ֻ�����У����мס��ҡ�������ʵ�飬����������ݣ�������CO2��ˮ�е��ܽ⣩��
�ش��������⣺
��1�������������������Ϊ______��
��2���������NaHCO3��KHCO3��������Ϊ______��
����֪��������A��NaHCO3��KHCO3��MgCO3��CaCO3���������е����ֻ�϶��ɣ�ͨ������������ش��������⣮
��1��ȡA�����������ᷴӦ����A������Ϊ��ֵʱ����������ɳɷֵĺ�����α仯�����ɵ����������Ϊ��ֵ����A�Ŀ�������ǣ����Բ�������Ҳ�ɲ��䣩
��2����A��MgCO3��CaCO3��ɵĻ���ȡ10.0�˵�A�����������ᷴӦ���������������x��Χ�Ƕ��٣�
| ʵ����� | �� | �� | �� |
| �����������g�� | 9.20 | 15.7 | 27.6 |
| ����CO2��������g�� | 4.4 | 6.6 | 6.6 |
��1�������������������Ϊ______��
��2���������NaHCO3��KHCO3��������Ϊ______��
����֪��������A��NaHCO3��KHCO3��MgCO3��CaCO3���������е����ֻ�϶��ɣ�ͨ������������ش��������⣮
��1��ȡA�����������ᷴӦ����A������Ϊ��ֵʱ����������ɳɷֵĺ�����α仯�����ɵ����������Ϊ��ֵ����A�Ŀ�������ǣ����Բ�������Ҳ�ɲ��䣩
| ������ | ������ | ������ | |
| A����� | ______ | ______ | ______ |
 ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У�����50��ijŨ�ȵ�ϡ���ᣮ��ַ�Ӧ��ȡ��ʣ����壬�����ˡ�����Ȳ����������ʣ��������������������������ϵ��ͼ��
ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У�����50��ijŨ�ȵ�ϡ���ᣮ��ַ�Ӧ��ȡ��ʣ����壬�����ˡ�����Ȳ����������ʣ��������������������������ϵ��ͼ��