��Ŀ����
 ��2011?տ������ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3?xH2O����E��F��IΪ��ɫ���壮����ͼʾ�ش��������⣮
��2011?տ������ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3?xH2O����E��F��IΪ��ɫ���壮����ͼʾ�ش��������⣮��1��д���й����ʵĻ�ѧʽ��B��
Ca��OH��2
Ca��OH��2
��C��H2O
H2O
����2��д����Ӧ�ܵĻ�ѧ����ʽ��
CaCl2+Na2CO3=CaCO3��+2NaCl
CaCl2+Na2CO3=CaCO3��+2NaCl
����3����Ӧ������
�û�
�û�
��Ӧ���Ӧ���ͣ�����4���ճ�������Ϊ����ֹ��Ӧ�۷�����ͨ����ȡ�Ĵ�ʩ��
ˢ���ᡢͿ�͵�
ˢ���ᡢͿ�͵�
��дһ��������5��Ҫʹ��Ӧ���ܹ���������Ҫ��Ӧ��B��M������M�Ļ�ѧʽΪ
NH4Cl
NH4Cl
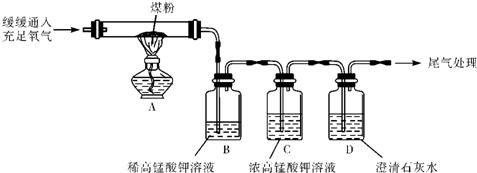
������������������Ϥ��һЩ���ʵĻ�ѧ���ԣ�������㣬θҺ�������ᣬ������������������ʯ�ң���ͨ�������·�Ӧ��������ģ����dz��н���ѧ��ֻ��ˮ�������������ɱ����ͻ�ƿڣ�������Щ֪ʶ�㣬���ǿ�����ɸ��⣮
����⣺���ݷ�Ӧ���������dz����ж�C Ϊˮ����ôE��F�������������������ݴ�F��G�������������֪GΪ�������Ҫ�ɷ֣�����Ϊ��������������ˮ������������ģ���ôF����������J������C Ϊˮ����Ҳ��֤������������жϣ�
F����������ôE ���������������Ѿ�֪��J�����ˣ���ôA�������ᣬ����������Ǻϣ�B���Ը����������������dz����ж���Ϊ�������ƣ��������������ᷴӦ�����Ȼ��ƺ�ˮ��CΪˮ����ôD �����Ȼ��ƣ�D������̼������Һ��Ӧ���ɰ�ɫ����H����Ҳ��һ����֤��B��D����ôH����̼��ƣ�
BΪ�������ƣ�������������֪��B������M��Ӧ����D�Ȼ��ƺ�Cˮ�Լ�I����������ѧ֪ʶ�ܹ��ͼӦ�����������ʵ����ʾ�����Σ������ɵ����������Ȼ��ƣ����ǰ�������ֽⷴӦ���軹ԭ�Ϳ��Եó�MΪ�Ȼ�泥�
����Ϊֹ�����ǰ����е����ʶ��������ж��������ܹ���Բ��˵���Ϳ���������ˣ�
����Ĵ�Ϊ����1��Ca��OH��2 H2O
��2�� CaCl2+Na2CO3=CaCO3��+2NaCl
��3����Ӧ������������ķ�Ӧ��Fe+2HCl=FeCl2+H2��Ϊ�û���Ӧ�����Դ�Ϊ���û�
��4����ֹ������Ĵ�ʩ��Ҫ���������ٱ��ֱ������ ���������Ϳ�ϱ����㣬�����г����Ĵ�ʩ����ˢ�ᡢͿ�͡��������������ȣ��ʴ�Ϊ��ˢ�ᡢͿ�͡��������������ȣ�
��5��������������֪ M�Ļ�ѧʽΪ��NH4Cl
F����������ôE ���������������Ѿ�֪��J�����ˣ���ôA�������ᣬ����������Ǻϣ�B���Ը����������������dz����ж���Ϊ�������ƣ��������������ᷴӦ�����Ȼ��ƺ�ˮ��CΪˮ����ôD �����Ȼ��ƣ�D������̼������Һ��Ӧ���ɰ�ɫ����H����Ҳ��һ����֤��B��D����ôH����̼��ƣ�
BΪ�������ƣ�������������֪��B������M��Ӧ����D�Ȼ��ƺ�Cˮ�Լ�I����������ѧ֪ʶ�ܹ��ͼӦ�����������ʵ����ʾ�����Σ������ɵ����������Ȼ��ƣ����ǰ�������ֽⷴӦ���軹ԭ�Ϳ��Եó�MΪ�Ȼ�泥�
����Ϊֹ�����ǰ����е����ʶ��������ж��������ܹ���Բ��˵���Ϳ���������ˣ�
����Ĵ�Ϊ����1��Ca��OH��2 H2O
��2�� CaCl2+Na2CO3=CaCO3��+2NaCl
��3����Ӧ������������ķ�Ӧ��Fe+2HCl=FeCl2+H2��Ϊ�û���Ӧ�����Դ�Ϊ���û�
��4����ֹ������Ĵ�ʩ��Ҫ���������ٱ��ֱ������ ���������Ϳ�ϱ����㣬�����г����Ĵ�ʩ����ˢ�ᡢͿ�͡��������������ȣ��ʴ�Ϊ��ˢ�ᡢͿ�͡��������������ȣ�
��5��������������֪ M�Ļ�ѧʽΪ��NH4Cl
�������������ƶ��⣬����ѧ���ۺ�����֪ʶ����������������ؼ�����ͻ�ƿڣ��������ʵĻ�ѧ���ԣ�˳��������һ�Ƴ���

��ϰ��ϵ�д�
�����Ŀ
 ��2011?տ����Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���ͼ��Ԫ�����ڱ��е�һ���л�ȡ����Ϣ����ȷ ���ǣ�������
��2011?տ����Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���ͼ��Ԫ�����ڱ��е�һ���л�ȡ����Ϣ����ȷ ���ǣ�������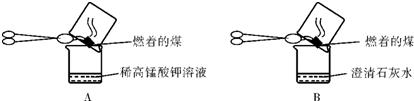
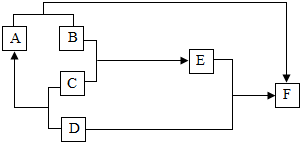 ��2011?���ޣ���ͼ��ijЩ��������֮��ת����ϵ����֪DΪ�ؿ��к������Ľ���Ԫ�صĵ��ʣ�BΪ�����C��һ��ϡ�ᣬ��Ũ��Һ��ˮϡ��ʱ��ų��������ȣ�EΪ��ɫ��Һ��A��F��Ϊ���ʣ��Իش��������⣨��ͼ�еķ�Ӧ��������Щ����������ȥ����
��2011?���ޣ���ͼ��ijЩ��������֮��ת����ϵ����֪DΪ�ؿ��к������Ľ���Ԫ�صĵ��ʣ�BΪ�����C��һ��ϡ�ᣬ��Ũ��Һ��ˮϡ��ʱ��ų��������ȣ�EΪ��ɫ��Һ��A��F��Ϊ���ʣ��Իش��������⣨��ͼ�еķ�Ӧ��������Щ����������ȥ����